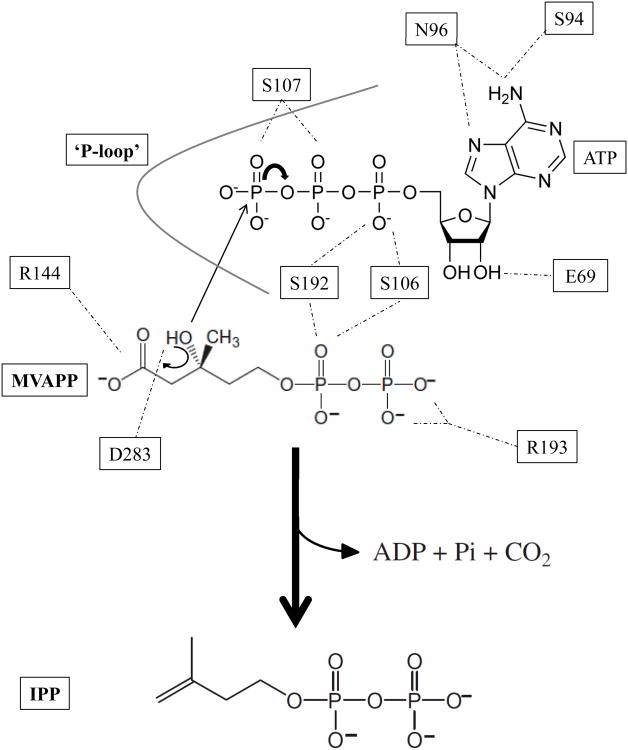
Figure 8.
Proposed scheme for the catalytic mechanism of mevalonate diphosphate decarboxylase. Abstraction of the C3-hydroxyl proton from MVAPP is aided by the carboxyl group of Asp283 which functions as the catalytic base in the MDD reaction. Several MDD amino acid side chains within the ‘P-loop’ interact with the ATP phosphoryl groups. Ser107 and Ser141 play prominent roles in stabilizing the γ -phosphoryl group. The guanidinium group of Arg144 interacts with the MVAPP carboxylate group assisting in decarboxylation after formation of the carbocation intermediate. Not all ligand interactions within the ternary crystal structure are depicted. Hydrogen bonds are shown as dashed lines.