Abstract
Streptococcus suis serotype 2 (SS2) is widely recognized in the veterinary world as the cause of rapidly progressive and fatal sepsis in infant pigs, manifested with meningitis, polyarthritis and pneumonia. It has evolved into a highly infectious strain, and caused two large-scale outbreaks of human epidemic in China, characterized bytypical toxic-shock syndrome and invasive infection. However, the molecular basis of virulence of this emerging zoonotic pathogen is still largely unknown. The present study shows that the sequence type (ST)7 epidemic strain S. suis 05ZYH33 causes higher mortality, higher necrosis of polymorphonuclear neutrophils and a significantly higher damage to human umbilical vein endothelial cells compared to the non-epidemic strain S. suis 1940. These differences appear to associate with the enhanced secretion of suilysin (sly) by S. suis 05ZYH33 compared to the non-epidemic strain 1940. Inclusion of additional strains confirmed that the epidemic ST7 strains produce more sly protein (mean, 1.49 g/ml; range, 0.76–1.91 g/ml) than non-epidemic strains (mean, 0.33 g/ml; range, 0.07–0.94 g/ml), and this difference is significant (P<0.001). The nonpolar mutant strain S. suis Δsly, constructed from the epidemic ST7 strain S. suis 05ZYH33 confirmed the role of sly on the enhanced virulence of S. suis ST7 strains. These findings suggest that increased sly production in S. suis 05ZYH33 facilitates penetration to the epithelium and its survival in the bloodstream, thereby contributing to the invasive infection.
Keywords: suilysin, invasive infection, epidemic strain, Streptococcus suis
Introduction
Streptococcus suis is an important emerging human threat that can cause severe systemic infection (1–3). Since the first reported cases of human infection by S. suis in 1968, the number of cases has significantly increased in the past few years, reaching ~1,000 cases by now, with the majority occurring in Southeast Asia. S. suis infections are the third most common cause of community-acquired bacterial meningitis in Hong Kong, and the leading cause of adult meningitis in Vietnam (4–5).
The S. suis serotype 2 (SS2) is the most common cause of the disease in humans, although serotypes 1, 4, 14 and 16 have also been reported to cause severe disease in a limited number of individuals. Most previous studies have concerned sporadic cases of S. suis infection, but two Chinese outbreaks (in 1998 and 2005) that involved >200 cases and 50 deaths, emphasized the importance of S. suis as an emerging zoonotic pathogen (6,7). The most important feature of these outbreaks was the high incidence of systemic disease, the proportionally few cases of meningitis, and the high mortality. Epidemiological surveys have indicated that all patients had a history of close contact with diseased pigs and pork-derived products, and that the emergence of the highly virulent SS2 strains played a key role in the severe outbreaks (8,9).
The current understanding of the pathogenesis of S. suis remains limited. Whole-genome sequencing and comparative genomic analysis (10) revealed that a DNA fragment of ~89 kb, designated as 89K, is present in the two Chinese strains (98HAH12 and 05ZYH33), and absent in the P1/7, which is a widely epidemic virulent Streptococcus suis serotype 2 strain. The 89K fragment was proposed to be a pathogenicity island, and genetic studies indicated that disruption of the Salk/SalR two-component system (TCS) inside the 89K considerably attenuates the virulence of the pathogen, whereas functional complementation restores virulence in infection experiments of piglets (11). A recent study (12) showed that 89K can spontaneously excise to form an extrachromosomal circular product, and the 89K excision intermediate can act as a substrate for lateral transfer to non-89K SS2 recipients via a genomic island type IV secretion system (T4SS) encoded in 89K. The authors proposed that these genetic events are important for the emergence, pathogenesis and persistence of epidemic SS2 strains. Based on sequence typing (ST), it was also suggested that ST7 strains are prevalent in China and possess a stronger capacity to stimulate T cells, naive T cells and peripheral blood mononuclear cell proliferation compared to ST1 strains (13–15). The authors of these studies proposed a two-stage hypothesis to explain streptococcal toxic shock syndrome in the Chinese outbreaks. However, both hypotheses need to be further investigated in the future.
Pore-forming toxins (PFTs) are the most prevalent virulence factors produced by disease-causing bacteria, and are required for the virulence of numerous important human pathogens, including Staphylococcus aureus, S. pyogenes, Clostridium perfringens and Aeromonas hydrophilia (16). A PFT produced by S. suis known as suilysin (sly) has been recognized as a virulence factor owing to its toxicity to the host epithelial cells, endothelial cells and macrophages (17–19). The sly protein belongs to a family of thiol-activated toxins and has a molecular weight of 54 kDa. It is a cholesterol-dependent cytolysin related to the streptolysin O of S. pyogenes and to pneumolysin of S. pneumoniae. Immunization to sly provides protection against lethal challenge with a serotype 2 strain in both mice and pig modelss (20,21). However, the expression profile of sly in certain S. suis populations and its role in causing invasive infections have not been characterized. In this study, we found that increased production of sly in the S. suis strain 05ZYH33 is associated with enhanced severity of S. suis infections and thus, may contribute to the translocation of the pathogen across the epithelial barrier. These results indicate that enhanced sly production may play an important role during S. suis invasive infections.
Materials and methods
Ethics
CD1 mice were obtained from the Experimental Animal Centre of the Academy of Military Medical Sciences (Beijing, China). Animal welfare and experimental procedures were approved by the Academy of Military Medical Sciences Animal Care and Use Committee, and were conducted in strict accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council of USA, 1996). Efforts were made to minimize animal suffering and to reduce the number of animals used. For the survival experiments, the decisions were made following the relevant guidelines of the Organisation for Economic Co-operation and Development [OECD Environmental Health and Safety Publications, Series on Testing and Assessment no. 19, ENV/JM/MONO(2000)7].
Bacterial strains and growth conditions
The bacterial strains used in this study are listed in Table I. S. suis was grown in Difco™ Todd-Hewitt broth (THB; Becton, Dickinson and Company, Franklin Lakes, NJ, USA) at 37°C. For antibiotic selection, 5 μg/ml of chloramphenicol (Cm) or 100 μg/ml spectinomycin (Spc) were used.
Table I.
Bacterial strains and plasmids used in this study.
| Material | Description | Area (year)-Origin | Status | Source/Ref. |
|---|---|---|---|---|
| Streptococcus suis strains | ||||
| Δsly | In frame deletion of sly in 05ZYH33 | Beijing, China (2011) - Our lab | Mutant | This study |
| 1330 | Sly−89K− | Canada-HPL | Avirulent | (25) |
| 05ZYH33 | Sly+89K+ | Sichuan, China (2005)-HP | Epidemic | This study |
| 98001 | Sly+89K+ | Jiangsu, China (1998)-HP | Epidemic | This study |
| 99001 | Sly+89K+ | Jiangsu, China (1998)-HP | Epidemic | This study |
| 98003 | Sly+89K+ | Jiangsu, China (1998)-HP | Epidemic | This study |
| 98005 | Sly+89K+ | Jiangsu, China (1998)-HP | Epidemic | This study |
| 98012 | Sly+89K+ | Jiangsu, China (1998)-HP | Epidemic | This study |
| 98015 | Sly+89K+ | Jiangsu, China (1998)-HP | Epidemic | This study |
| 98242 | Sly+89K+ | Jiangsu, China (1998)-HP | Epidemic | This study |
| 4 | Sly+89K+ | Sichuan, China (2005)-DP | Epidemic | This study |
| 5 | Sly+89K+ | Sichuan, China (2005)-DP | Epidemic | This study |
| SUN | Sly+89K+ | Jiangsu, China (2006)-HP | Epidemic | This study |
| 606 | Sly+89K− | China (1980)-DP | Non-epidemic | This study |
| 607 | Sly+89K− | Japan-DP | Non-epidemic | This study |
| 1940 | Sly+89K− | China (1980)-DP | Non-epidemic | This study |
| 1941 | Sly+89K− | China (1980)-DP | Non-epidemic | This study |
| NJ | Sly+89K− | Jiangsu, China-DP | Non-epidemic | This study |
| 4005 | Sly+89K− | The Netherlands-DP | Non-epidemic | (26) |
| s735 | Sly+89K− | The Netherlands-DP | Non-epidemic | (27) |
| T15 | Sly+89K− | Europe-HPL | Non-epidemic | (28) |
| Plasmids | ||||
| pCR2.1 | na | na | na | Invitrogen™ |
| pSET4s | na | na | na | (29) |
Sly, suilysin; 89K, 89 kb pathogenicity island; HPL, healthy piglets; HP, human patients; DP, diseased piglets; na, not applicable.
Cell culture and lactate dehydrogenase (LDH) release assay
Human umbilical vein endothelial cells (HUVECs; Institute of Biochemistry and Cell Biology, Shanghai, China) were cultured in RPMI-1640 medium containing 10% fetal bovine serum (FBS). Cells (200 μl) were added at a density of 5×104 cells/ml into 96-well culture plates. S. suis strains 05ZYH33 or 1940 at the mid-log phase of growth were resuspended in cell culture medium and were added to the cell culture wells at multiplicity of infection (MOI) of 100. The amount of released lactate dehydrogenase (LDH) from HUVECs was calculated with an LDH release assay kit (Promega, Madison, WI, USA) after 4 h of incubation.
Targeted mutagenesis of the sly gene
Polymerase chain reaction (PCR) was used to generate an in-frame substitution of the sly gene with the Cm gene using a previously described method (22). Briefly, 644 and 590 bp immediately upstream and downstream of the sly coding sequence (SSU05_1403) were amplified with the primer pairs sly_upF/_upR and sly_downF/_downR, respectively. The Cm gene was amplified using the primer pair Cm_F/_R (Table II). The PCR products were cloned into the Invitrogen™ pCR2.1 plasmid (Thermo Fisher Scientific, Waltham, MA, USA), digested using restriction enzymes and subcloned into the plamsid pSET4s one by one. Allelic exchange mutagenesis in S. suis 05ZYH33 was performed as previously described (22) to generate the mutant Δsly. Allelic replacement of sly with Cm in the S. suis chromosome was confirmed by PCR and sequence analysis using the Phusion High-Fidelity DNA Polymerase reagents (New England Biolabs, Ipswich, MA, USA) on a ABI 3730XL machine (Applied Biosystems, Carlsbad, CA, USA).
Table II.
Oligonucleotide primer sequences used in this study.
| Primer | Sequence (5′-3′) | Restriction enzyme |
|---|---|---|
| Sly_upF | GGGGGAAGCTTCTAGTCGGGGGAGTTTTTGTG | HindIII |
| Sly_upR | GGGGGGGTCGACTTAATATCTTGTTTGGAATCTG | SalI |
| Cm_F | GCGGTCGACTAATTCGATGGGTTCCGAGG | SalI |
| Cm_R | CGCGGATCCCACCGAACTAGAGCTTGATG | BamHI |
| Sly_downF | GGGGGGATCCCATGGGAGTGGTGGAGAACAGT | BamHI |
| Sly_downR | GGGGGGGGAATTCTTGGCCCGAATACCGACAG | EcoRI |
Sly, suilysin; up, upstream; down, downstream; F, forward; R, reverse; Cm, chloramphenicol; underlined, restriction sites.
Enzyme-linked immunosorbent assay (ELISA) assay
The sly level in the supernatant of different S. suis strain cultures was determined using a double antibody sandwich ELISA assay. The antibodies used were polyclonal. Briefly, purified rabbit anti-sly IgG (1:5,000 dilution) was used to coat the wells of a microtiter plate. Purified rat anti-sly IgG (1:5,000) was used as the primary antibody, peroxidase-conjugated goat anti-rat antibody (1:5,000) was used as the secondary antibody, and K-Blue was used as the substrate (all from Santa Cruz Biotechnology, Santa Cruz, CA, USA). After 10 min of incubation at room temperature, the reaction was terminated with addition of 2 N H2SO4, and the optical density was measured at 450 nm (OD450) using the SpectraMax® Plus384 Absorbance Microplate reader (Molecular Devices LLC, Sunnyvale, CA, USA). Purified sly protein (23) was used to establish the standard curve.
Hemolytic activity assay
The haemolytic activity assay was performed using a previously described method (24). Briefly, the culture supernatant of S. suis strains was diluted 2-fold in phosphate-buffered saline (PBS). An equal amount of human red blood cells (RBCs), obtained from the Department of Hematology (Hospital 307 of Chinese People’s Liberation Army, Beijing China) and washed twice in PBS, was added to 0.5 ml of each dilution (final concentration of RBCs, 1.4%). Following incubation for 1 h at 37°C, the mixtures were sedimented by centrifugation (1,500 × g for 10 min), and the supernatants were transferred to microplates. Following correction with the controls, which lacked either haemolysin or erythrocytes, the OD540 of the supernatant of each dilution was measured on a SpectraMax Plus Absorbance Microplate reader.
Reverse transcription-quantitative-PCR (RT-qPCR) analysis
The RT-qPCR analysis was performed with a sly gene-specific primer set (forward, ACTTACGAGCCACAAGAGATTC and reverse, GCAGCCTTAGCATCAATAACAG) on cDNA from the S. suis strains 05ZYH33 and 1940. The assays were carried out in triplicate using reagents of the SYBR Premix Ex Taq™ mastermix (Takara Bio, Co., Ltd., Shiga, Japan) on an Opticon 2 system (MJ Research, Waltham, MA, USA) using RNA isolated from three independent cultures for each strain. RNA was extracted using the TRIzol RNA Reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) following the manufacturer’s instructions. The PCR cycling conditions were as follows: 95°C for 10 min followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. The gene encoding the 16S rRNA was used as an internal control. The Ct values were normalized to the average Ct (30) and the mean fold-changes for the target genes were calculated as described by Livak and Schmittgen (31).
Polymorphonuclear neutrophil (PMN) phagocytosis assay
The PMN phagocytosis assay was performed as previously described (32). Human PMNs were isolated from healthy human blood as follows: blood was incubated for 40 min at room temperature in a ratio of 3:1 ratio of 6.0% Dextran to sediment erythrocytes. Ficoll, 70% percoll and blood supernatant were combined (1:1:1) and centrifuged at 400 × g for 30 min at room temperature to separate PMNs. The PMNs were aspirated in to a new cuvette, resuspended in 1640-RPMI, and enumerated by microscopy. Briefly, 1 ml of human PMNs (107 cells) were mixed with an equal number of pre-opsonized S. suis colonies and incubated for 15 min at 37°C under continuous rotation to allow phagocytosis. When needed, different concentrations of recombinant sly protein (23) were added (50–500 ng). To quantify the intracellular bacteria, extracellular S. suis were killed by incubating with 100 μg/ml gentamicin and 5 g/ml penicillin for 1 h at 4°C. Subsequently, the cells were washed twice with PBS and lysed for 15 min at room temperature in 400 μl of 1% saponin. The viable bacteria were counted on an inverted IX81 microscope (Olympus, Tokyo, Japan) after plating serial dilutions of the supernatants on THB agar plates.
Mouse infections
CD1 female mice (6-week-old) were chosen to evaluate the virulence of the S. suis strains 05ZYH33 and 1940. Briefly, mice (n=10 per group) were intraperitoneally injected with 108 living colony forming units (CFUs) of S. suis strains 05ZYH33 and 1940 in 0.1 ml sterile saline. The mortality of mice infected with these bacterial strains was recorded at 4-h intervals for 32 h. To count the bacteria present in the blood, blood samples were collected from the tail vein and plated onto THB agar to accurately determine the viable bacteria at 5 h post-infection. To assess the necrosis of PMNs, we sacrificed the mice and injected 5 ml of RPMI-1640 medium containing 10% FBS into the abdominal cavities. Then, mice were surgically opened and 4-ml exudates were collected with 23-G needles. The exudates were centrifuged at 500 × g for 5 min, and cell pellets were resuspended in 100 ml of staining buffer (PBS containing 1% goat serum). The samples were stained either with fluorescein isothiocyanate (FITC)-conjugated anti-mouse anti-Ly-6G (a neutrophil marker), or with the appropriate isotype control antibody (eB149/10H5; both from eBiosciences, San Diego, CA, USA). Propidium iodide (0.5 mg/ml; BD Biosciences, San Jose, CA, USA) was used to determine the cell viability. Samples were analyzed on a FACSCalibur flow cytometer and the necrotic PMNs were quantified with the CellQuest software (both from BD Biosciences). Data were obtained from 30,000 cells for each sample.
CD1 female mice (6-week-old, ~22 g) were chosen to evaluate the virulence of the S. suis strain 05ZYH33 and the Δsly mutant. Mice (n=10, per group) were intraperitoneally injected with 0.5 ml (~5×108 CFUs) of S. suis at the late-exponential growth phase in THB medium. The mortality of the infected mice was recorded for 50 h, and the bacteria in the blood were counted at different time-points post-infection. To histopathologically assess the severity of changes in the abdominal wall tissues, mice were surgically opened 6 h after injection. The abdominal wall tissues were fixed in 10% Formaldehyde (Sigma, St. Louis, MO, USA), sectioned and stained with hematoxylin and eosin. The slides were observed and photographed using a BX53 fluorescence microscope (Olympus, Tokyo, Japan) equipped with a DP72 CCD camera.
Statistical analysis
Statistical analysis was performed using the SPSS 13.0 version software (SPSS Inc., Chicago, IL, USA). Statistical significance of differences was assessed with Student’s t-tests, with a p-value <0.05 considered to indicate statistically significant differences.
Results
The S. suis epidemic strain is more virulent than the non-epidemic strain
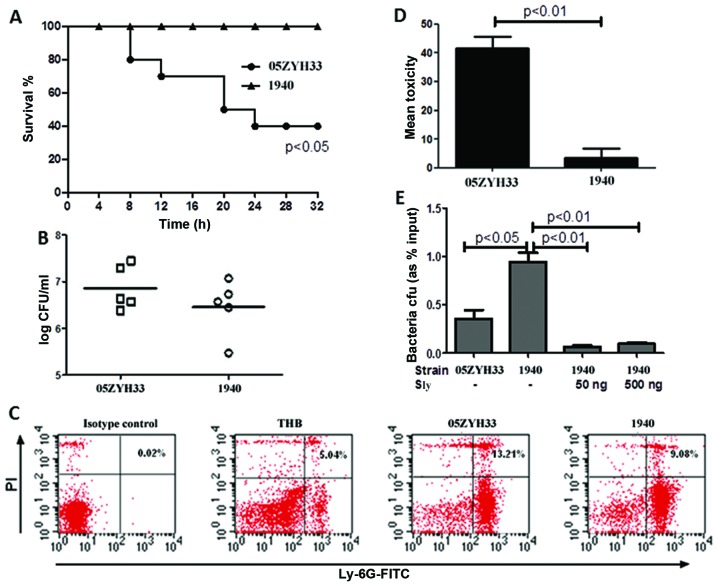
The S. suis epidemic strains are expected to show high virulence, and need to resist host defence mechanisms and be able to invade the epithelial or the endothelial barrier in order to cause successful infection. To evaluate the potential differences in virulence between S. suis epidemic and non-epidemic strains, CD1 female mice were intraperitoneally injected with 108 CFUs of the S. suis strains 05ZYH33 (epidemic, ST7) or 1940 (non-epidemic) in 0.1 ml sterile saline. The strain 05ZYH33 caused significantly higher mortality (p<0.05) than the 1940 strain (Fig. 1A). The bacterial concentration in the blood of the infected mice also appeared different at 5 h post-infection, although the difference was not statistically significant (Fig. 1B). In addition, S. suis 05ZYH33 caused higher necrosis of PMNs (Fig. 1C), the major cell type mediating acute inflammatory responses to bacterial infections, compared to S. suis 1940 (13.21±3.11 vs. 9.08±2.56%). Next, we used HUVECs to test the ability of the two S. suis strains to damage the cells. The results showed that 05ZYH33 has a higher cytotoxicity than 1940, as determined by the LDH assay (Fig. 1D). Taken together, these data demonstrate that the S. suis epidemic strain 05ZYH33 is more virulent than the non-epidemic strain 1940.
Figure 1.
Streptococcus suis epidemic strain 05ZYH33 is more virulent than the non-epidemic strain 1940. (A) CD1 female mice were intraperitoneally injected with 108 CFUs of living S. suis 05ZYH33 and 1940 in 0.1 ml sterile saline. The mortality of mice was recorded at 4-h intervals for 32 h. Cumulative results from three independent experiments, each with 10 animals per group, are shown. (B) Bacteria in the blood (n=5, per group) were counted at 5 h post-infection. (C) The necrosis of PMNs in mice infected by different bacterial strains was assayed using fluorescein isothiocyanate (FITC)-conjugated anti-mouse anti-Ly-6G and propidium iodide (PI) staining. Data represent the values obtained in four independent experiments. THB, Todd-Hewitt broth. (D) S. suis 05ZYH33 or 1940 (108 CFUs) were incubated with human umbilical vein endothelial cells (MOI =100) for 4 h. Cytotoxicity was determined by the lactate dehydrogenase release assay. The data represent the mean ± standard deviation from three independent experiments. (E) Pre-opsonised S. suis 05ZYH33 or 1940 (107 CFUs) were incubated with purified human polymorphonuclear neutrophils (MOI =1) in the presence or absence of recombinant sly protein for 15 min. The number of bacteria phagocytized by the PMNs was counted.
Next, we investigated whether the sly protein may contribute to the enhanced virulence of the S. suis epidemic strain. Pre-opsonised S. suis 05ZYH33 or 1940 were incubated with purified human PMNs (MOI =1) for 15 min in the presence or absence of the sly protein, and the bacteria phagocytized by PMNs were counted. This assay showed that PMNs phagocytized the S. suis 1940 strain at higher rates compared to the 05ZYH33 strain, (Fig. 1E) while adding sly protein significantly decreased the phagocytosis of S. suis 1940 by the PMNs.
The S. suis epidemic strain 05ZYH33 shows increased production of sly compared to the non-epidemic strains
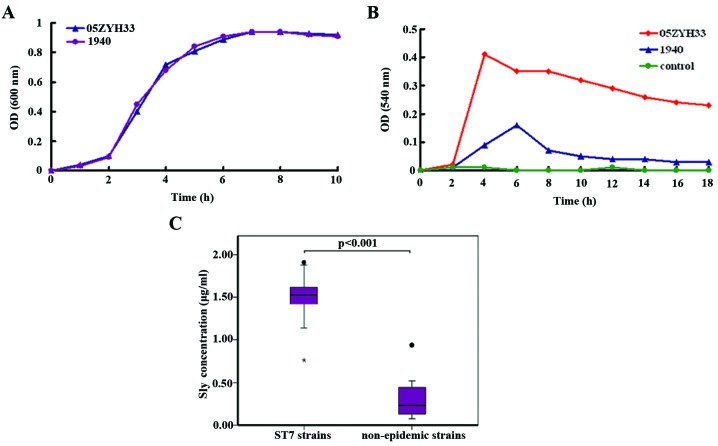
Since the sly protein displays hemolytic activity, we then compared the relative amounts of sly produced by the S. suis epidemic strain 05ZYH33 and the non-epidemic strain 1940. Although both strains showed the same growth rate (Fig. 2A), the supernatant of S. suis 05ZYH33 displayed higher hemolytic activity than that of S. suis 1940 (Fig. 2B). This phenotypic difference was further confirmed by qRT-PCR, which indicated a difference in sly expression between the two S. suis strains; a 6.3-fold difference at the mRNA transcription level of sly was observed between the two strains (data not shown).
Figure 2.
Streptococcus suis ST7 epidemic strains show increased production of suilysin (sly) compared to non-epidemic strains. S. suis 05ZYH33 and 1940 (A) growth curves and (B) relative hemolytic activities, assessed in the supernatants collected at different time-points. (C) Sly protein concentration, determined by an enzyme-linked immunosorbent assay, performed on 11 ST7 strains and 8 non-epidemic strains listed in Table I.
In order to confirm the hypothesis that the S. suis ST7 epidemic strains may have increased production of sly compared to the non-epidemic strains, 11 S. suis ST7 epidemic strains and eight non-epidemic strains were collected (Table I), and the amount of sly was determined in the supernatant at 7 h of culture, using a sandwich ELISA assay (Fig. 2C). The epidemic ST7 strains produced more sly (mean, 1.49 g/ml; range, 0.76–1.91 g/ml) than the non-epidemic strains (mean, 0.33 g/ml; range, 0.07–0.94 g/ml). Moreover, the difference between the two groups was significant (p<0.001).
Sly is associated with enhanced severity of S. suis infections
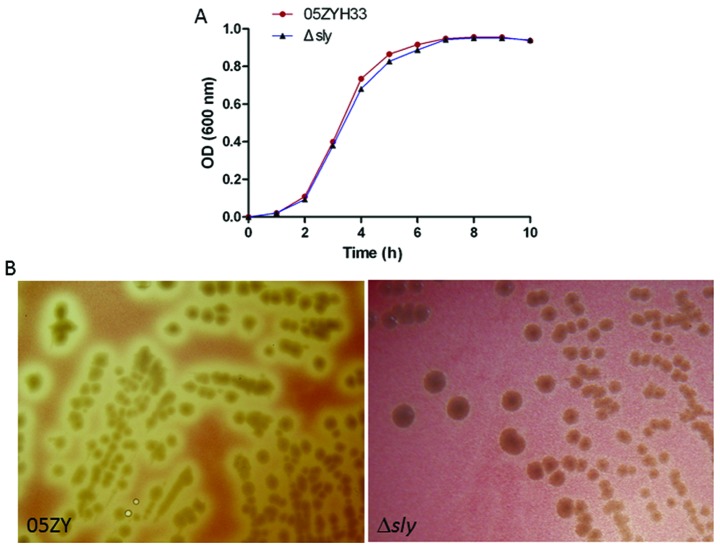
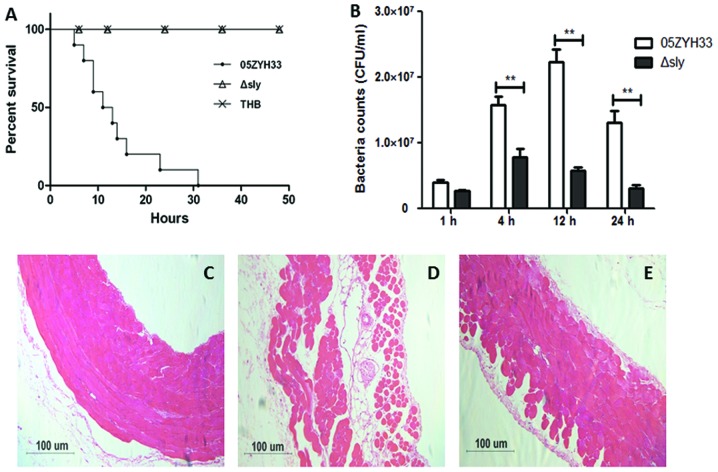
To further explore the roles of sly in invasive infection of the 05ZYH33 strain, the sly gene was in-frame substituted by the Cm gene in the S. suis 05ZYH33 strain, to generate the nonpolar mutant Δsly. The mutant Δsly strain showed reduced hemolytic activity, but similar growth rates compared to the wild-type strain S. suis 05ZYH33 (Fig. 3). We further investigated the virulence of this mutant. CD1 mice (6-week-old female, ~22 g) were infected by intraperitoneal injection with 0.5 ml (~5×108 CFUs) S. suis at the late-exponential growth phase in THB. The mortality of the infected mice was recorded for 50 h. No mice died in the Δsly or the THB control group, while the mice infected with S. suis 05ZYH33 all died within 32 h post-infection. The bacterial concentration in the blood was evaluated at different time-points post-infection (Fig. 4A). The parental 05ZYH33 strain survived/multiplied in the blood at higher rates than Δsly at 4, 12 and 24 h (Fig. 4B). Histopathological examination of the abdominal wall tissues was also performed 6 h after the infection. The abdominal epithelial cells of mice infected by S. suis 05ZYH33 lost their normal morphological features and the integrity of the tight junctions, while the epithelial cells infected by S. suis Δsly showed a few changes (Fig. 4C–E). Taken together, these data demonstrate that sly is associated with enhanced severity of S. suis 05ZYH33 infections.
Figure 3.
The mutant Δsly strain shows (A) similar growth rates compared to the wild-type strain S. suis 05ZYH33, but (B) reduced hemolytic activity.
Figure 4.
Suilysin is associated with the enhanced severity of S. suis 05ZYH33 infections. CD1 mice (6-week-old, female, ~22 g) were intraperitoneally injected with 0.5 ml (~5×108 CFUs) S. suis at the late-exponential growth phase, supplemented with Todd-Hewitt broth (THB). (A) The mortality of the infected mice, recorded for 50 h. (B) The bacterial count in the blood at different time-points post-infection. The abdominal wall tissues were histopathologically examined 6 h after injection of (C) THB, (D) 05ZYH33, and (E) Δsly, using hematoxylin and eosin staining. Images are representative of two independent experiments.
Discussion
S. suis is a swine pathogen responsible for a number of infections, including meningitis, endocarditis and septicemiae, and is also an important zoonotic agent. The two outbreaks that occurred in China in 1998 and 2005 affected the world perspective regarding the threat that this pathogen represents for humans. Subsequent investigations on S. suis at the molecular and the genomic level confirmed that the emergence of the highly virulent SS2 strains, or epidemic ST7 strains, played a key role in these severe outbreaks (13). In the present study, we sought to determine the capacity of the S. suis protein sly to enhance the virulence of the epidemic ST7 strain 05ZYH33.
A previous study showed that S. suis ST7 strains express the proposed virulence markers muramidase-released protein (MRP), extracellular protein factor (EF), and sly (15). However to date, there is no study that specifically focused on how the differences in the expression abundance of bacterial virulence factors may affect the severity of S. suis infections. We hereby showed, using an animal model, that the S. suis ST7 epidemic strain 05ZYH33 causes higher mortality, higher necrosis of PMNs and significantly higher damage to HUVECs than the S. suis non-epidemic strain 1940. In agreement with these results, epidemic ST7 strains produced more sly than non-epidemic strains. This significant difference in sly secretion may be of outmost importance in S. suis 05ZYH33 infections. Phagocytosis is the first line of cellular defense against acute infectious diseases. A clinical association with invasive human infections implies that bacteria can survive the innate host defense responses raised in order to clear the bacteria from the bloodstream and the tissues. In other pathogenic streptococcal infections, hemolysins have been shown to be involved in bacteria-phagocyte interactions. For example, in Group B Streptococcus (GBS) infections, Liu et al (32) elegantly demonstrated the multi-factorial roles of the pore-forming toxin β-hemolysin/cytolysin (βH/C) in thwarting the immune phagocytic defenses. In the present study, we demonstrated that sly mediates inhibition of the phagocytic uptake by human PMNs in vitro. Moreover, we demonstrated that the S. suis strain with enhanced sly expression causes enhanced PMN necrosis in an in vivo mouse model. Taken together, these findings suggest that the sly-mediated inhibition of phagocytosis and cytolysis is directly associated with a mechanism impairing the function and viability of PMNs, and thus contributing to the enhanced survival of epidemic S. suis strains.
The ability of sly to enhance the severity of S. suis infection was confirmed using a mutant of S. suis 05ZYH33 deficient in sly expression. The survival rate of mice challenged by the Δsly strain was significantly increased, and the bacterial count in the blood decreased compared to the parental strain 05ZYH33. These data strongly support the contribution of sly in enhancing the severity of S. suis ST7 strain infections, and are consistent with the previous study of Allen et al (33).
In our intraperitoneal infection animal model, S. suis needs to successfully cross the abdominal epithelial barrier and survive in the bloodstream in order to cause invasive infection. Among the potential strategies that can allow this are: transcellular transport by passive or adhesion-induced transcytosis, paracellular passage through opened tight junctions, disruption of the barrier due to a direct cytotoxic effect, leukocyte-facilitated transport by infected phagocytes (34). Histopathological examination showed that the mice infected by S. suis 05ZYH33 lost their abdominal epithelial cell morphological features and the integrity of the tight junctions compared to mice infected by the mutant Δsly. The S. pneumoniae toxin pneumolysin recognizes Toll-like receptor (TLR)4 on dendritic cells (35), and S. pneumoniae and H. influenzae were shown to exploit TLR-dependent downregulation of claudins 7 and 10, tight junction key components for the maintenance of the epithelial barrier integrity(36), to facilitate translocation across the epithelium (37). However, in vivo studies carried out in our laboratory indicate that the S. suis sly protein contributes to the release of inflammatory cytokines in a TLR4-independent manner (data not shown). Whether activation of TLR4 by sly can facilitate the S. suis translocation across the epithelium needs to be further studied.
In conclusion, we have shown that differences in sly production are linked to differences in S. suis virulence. The pathogenic S. suis ST7 strain 05ZYH33 appeared to have an increased ability to subvert host clearance mechanisms, which may allow its survival and dissemination into the bloodstream. The cytotoxic effects of sly may inhibit the uptake of microorganisms by the host phagocytes and can directly cause epithelial cell damage, enhancing the microbial spread into deeper tissues. Thus, in a murine model, sly contributes to increased bacterial load and death. Further understanding of the precise molecular mechanisms underlying the sly-mediated pathogenicity may provide potential therapeutic targets for a better control of S. suis infections.
Acknowledgements
This study was supported by grants from the Natural Sciences Foundation of China (30870091 and 81171528). We thank Professor Henk J. Wisselink and Marcelo Gottschalk for kindly providing S. suis strains.
References
- 1.Lun ZR, Wang QP, Chen XG, Li AX, Zhu XQ. Streptococcus suis: an emerging zoonotic pathogen. Lancet Infect Dis. 2007;7:201–209. doi: 10.1016/S1473-3099(07)70001-4. [DOI] [PubMed] [Google Scholar]
- 2.Segura M. Streptococcus suis: an emerging human threat. J Infect Dis. 2009;199:4–6. doi: 10.1086/594371. [DOI] [PubMed] [Google Scholar]
- 3.Wertheim HF, Nghia HD, Taylor W, Schultsz C. Streptococcus suis: an emerging human pathogen. Clin Infect Dis. 2009;48:617–625. doi: 10.1086/596763. [DOI] [PubMed] [Google Scholar]
- 4.Hui AC, Ng KC, Tong PY, Mok V, Chow KM, Wu A, Wong LK. Bacterial meningitis in Hong Kong: 10-years’ experience. Clin Neurol Neurosurg. 2005;107:366–370. doi: 10.1016/j.clineuro.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Mai NT, Hoa NT, Nga TV, et al. Streptococcus suis meningitis in adults in Vietnam. Clin Infect Dis. 2008;46:659–667. doi: 10.1086/527385. [DOI] [PubMed] [Google Scholar]
- 6.Yu H, Jing H, Chen Z, et al. Human Streptococcus suis outbreak, Sichuan, China. Emerg Infect Dis. 2006;12:914–920. doi: 10.3201/eid1206.051194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gottschalk M, Segura M, Xu J. Streptococcus suis infections in humans: the Chinese experience and the situation in North America. Anim Health Res Rev. 2007;8:29–45. doi: 10.1017/S1466252307001247. [DOI] [PubMed] [Google Scholar]
- 8.Normile D. Infectious diseases. WHO probes deadliness of China’s pig-borne disease. Science. 2005;309:1308–1309. doi: 10.1126/science.309.5739.1308a. [DOI] [PubMed] [Google Scholar]
- 9.Ngo TH, Tran TB, Tran TT, et al. Slaughterhouse pigs are a major reservoir of Streptococcus suis serotype 2 capable of causing human infection in southern Vietnam. PloS one. 2011;6:e17943. doi: 10.1371/journal.pone.0017943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen C, Tang J, Dong W, et al. A glimpse of streptococcal toxic shock syndrome from comparative genomics of S. suis 2 Chinese isolates. PLoS One. 2007;2:e315. doi: 10.1371/journal.pone.0000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li M, Wang C, Feng Y, Pan X, et al. SalK/SalR, a two-component signal transduction system, is essential for full virulence of highly invasive Streptococcus suis serotype 2. PLoS One. 2008;3:e2080. doi: 10.1371/journal.pone.0002080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li M, Shen X, Yan J, et al. GI-type T4SS-mediated horizontal transfer of the 89K pathogenicity island in epidemic Streptococcus suis serotype 2. Mol Microbiol. 2011;79:1670–1683. doi: 10.1111/j.1365-2958.2011.07553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye C, Zhu X, Jing H, et al. Streptococcus suis sequence type 7 outbreak, Sichuan, China. Emerg Infect Dis. 2006;12:1203–1208. doi: 10.3201/eid1208.060232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye C, Bai X, Zhang J, et al. Spread of Streptococcus suis sequence type 7, China. Emerg Infect Dis. 2008;14:787–791. doi: 10.3201/eid1405.070437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye C, Zheng H, Zhang J, et al. Clinical, experimental, and genomic differences between intermediately pathogenic, highly pathogenic, and epidemic Streptococcus suis. J Infect Dis. 2009;199:97–107. doi: 10.1086/594370. [DOI] [PubMed] [Google Scholar]
- 16.Alouf JE. Molecular features of the cytolytic pore-forming bacterial protein toxins. Folia Microbiol (Praha) 2003;48:5–16. doi: 10.1007/BF02931271. [DOI] [PubMed] [Google Scholar]
- 17.Segura M, Gottschalk M. Streptococcus suis interactions with the murine macrophage cell line J774: adhesion and cytotoxicity. Infect Immun. 2002;70:4312–4322. doi: 10.1128/IAI.70.8.4312-4322.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lalonde M, Segura M, Lacouture S, Gottschalk M. Interactions between Streptococcus suis serotype 2 and different epithelial cell lines. Microbiology. 2000;146:1913–1921. doi: 10.1099/00221287-146-8-1913. [DOI] [PubMed] [Google Scholar]
- 19.Charland N, Nizet V, Rubens CE, Kim KS, Lacouture S, Gottschalk M. Streptococcus suis serotype 2 interactions with human brain microvascular endothelial cells. Infect Immun. 2000;68:637–643. doi: 10.1128/iai.68.2.637-643.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobs AA, Loeffen PL, van den Berg AJ, Storm PK. Identification, purification, and characterization of a thiol-activated hemolysin (suilysin) of Streptococcus suis. Infect Immun. 1994;62:1742–1748. doi: 10.1093/benz/9780199773787.article.b00034458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobs AA, van den Berg AJ, Loeffen PL. Protection of experimentally infected pigs by suilysin, the thiol-activated haemolysin of Streptococcus suis. Vet Rec. 1996;139:225–228. doi: 10.1136/vr.139.10.225. [DOI] [PubMed] [Google Scholar]
- 22.Pian Y, Gan S, Wang S, et al. Fhb, a novel factor H-binding surface protein, contributes to the antiphagocytic ability and virulence of Streptococcus suis. Infect Immun. 2012;80:2402–2413. doi: 10.1128/IAI.06294-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lv QY, Hao HJ, Bi LL, Zheng YL, Jiang YQ, Lv SX. Purification and biological activities analysis of streptococcus suis Serotype 2 suilysin. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2011;27:374–376. (In Chinese) [PubMed] [Google Scholar]
- 24.Gottschalk MG, Lacouture S, Dubreuil JD. Characterization of Streptococcus suis capsular type 2 haemolysin. Microbiology. 1995;141:189–195. doi: 10.1099/00221287-141-1-189. [DOI] [PubMed] [Google Scholar]
- 25.Berthelot-Hérault F, Gottschalk M, Morvan H, Kobisch M. Dilemma of virulence of Streptococcus suis: Canadian isolate 89–1591 characterized as a virulent strain using a standardized experimental model in pigs. Can J Vet Res. 2005;69:236–240. [PMC free article] [PubMed] [Google Scholar]
- 26.Wisselink HJ, Joosten JJ, Smith HE. Multiplex PCR assays for simultaneous detection of six major serotypes and two virulence-associated phenotypes of Streptococcus suis in tonsillar specimens from pigs. J Clin Microbiol. 2002;40:2922–2929. doi: 10.1128/JCM.40.8.2922-2929.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Martinez G, Gottschalk M, et al. Identification of a surface protein of Streptococcus suis and evaluation of its immunogenic and protective capacity in pigs. Infect Immun. 2006;74:305–312. doi: 10.1128/IAI.74.1.305-312.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wisselink HJ, Reek FH, Vecht U, Stockhofe-Zurwieden N, Smits MA, Smith HE. Detection of virulent strains of Streptococcus suis type 2 and highly virulent strains of Streptococcus suis type 1 in tonsillar specimens of pigs by PCR. Vet Microbiol. 1999;67:143–157. doi: 10.1016/s0378-1135(99)00036-x. [DOI] [PubMed] [Google Scholar]
- 29.Takamatsu D, Osaki M, Sekizaki T. Thermosensitive suicide vectors for gene replacement in Streptococcus suis. Plasmid. 2001;46:140–148. doi: 10.1006/plas.2001.1532. [DOI] [PubMed] [Google Scholar]
- 30.Schmittgen TD, Zakrajsek BA. Effect of experimental treatment on housekeeping gene expression: validation by real-time, quantitative RT-PCR. J Biochem Biophys Methods. 2000;46:69–81. doi: 10.1016/s0165-022x(00)00129-9. [DOI] [PubMed] [Google Scholar]
- 31.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 32.Liu GY, Doran KS, Lawrence T, Turkson N, Puliti M, Tissi L, Nizet V. Sword and shield: linked group B streptococcal beta-hemolysin/cytolysin and carotenoid pigment function to subvert host phagocyte defense. Proc Natl Acad Sci USA. 2004;101:14491–14496. doi: 10.1073/pnas.0406143101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allen AG, Bolitho S, Lindsay H, et al. Generation and characterization of a defined mutant of Streptococcus suis lacking suilysin. Infect Immun. 2001;69:2732–2735. doi: 10.1128/IAI.69.4.2732-2735.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Join-Lambert O, Morand PC, Carbonnelle E, Coureuil M, Bille E, Bourdoulous S, Nassif X. Mechanisms of meningeal invasion by a bacterial extracellular pathogen, the example of Neisseria meningitidis. Prog Neurobiol. 2010;91:130–139. doi: 10.1016/j.pneurobio.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Bernatoniene J, Zhang Q, Dogan S, Mitchell TJ, Paton JC, Finn A. Induction of CC and CXC chemokines in human antigen-presenting dendritic cells by the pneumococcal proteins pneumolysin and CbpA, and the role played by toll-like receptor 4, NF-kappaB, and mitogen-activated protein kinases. J Infect Dis. 2008;198:1823–1833. doi: 10.1086/593177. [DOI] [PubMed] [Google Scholar]
- 36.Dominguez-Punaro MC, Segura M, Plante MM, Lacouture S, Rivest S, Gottschalk M. Streptococcus suis serotype 2, an important swine and human pathogen, induces strong systemic and cerebral inflammatory responses in a mouse model of infection. J Immunol. 2007;179:1842–1854. doi: 10.4049/jimmunol.179.3.1842. [DOI] [PubMed] [Google Scholar]
- 37.Clarke TB, Francella N, Huegel A, Weiser JN. Invasive bacterial pathogens exploit TLR-mediated downregulation of tight junction components to facilitate translocation across the epithelium. Cell Host Microbe. 2011;9:404–414. doi: 10.1016/j.chom.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]