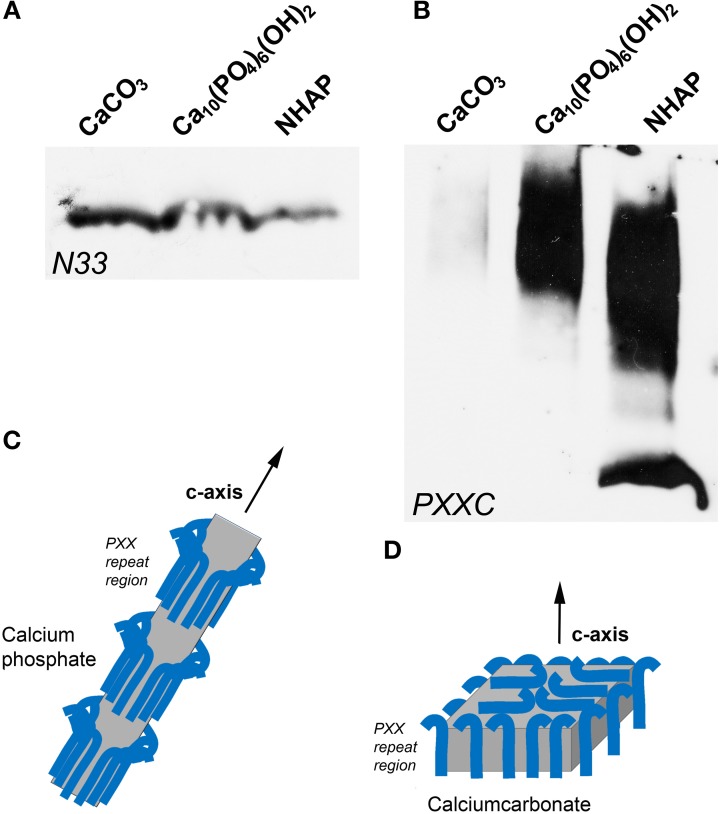
Figure 4.
Binding of amelogenin fragments to calcium minerals. (A,B) illustrate results from parallel experiments in which either an amelogenin N-terminal fragment (N33, A) or a C-terminus augmented amelogenin repeat fragment (PXXC, B) were incubated with three different calcium minerals, including calcium carbonate (CaCO3), calcium phosphate, and nanohydroxyapatite (NHAP). (C,D) illustrates our explanation for the differences in calcium mineral crystal growth when subjected to different protein environments. Here we propose that polyproline repeat regions (blue hooks) as they occur in amelogenin bind to the a- and b- axis of calcium phosphate crystals and allow for the growth of long apatite crystal through expansion mostly in c-axis direction (C). In contrast, proteins including polyproline peptides appear to affect calcium carbonate crystal growth equally in all directions, resulting in an overall compaction of the final crystal (D).