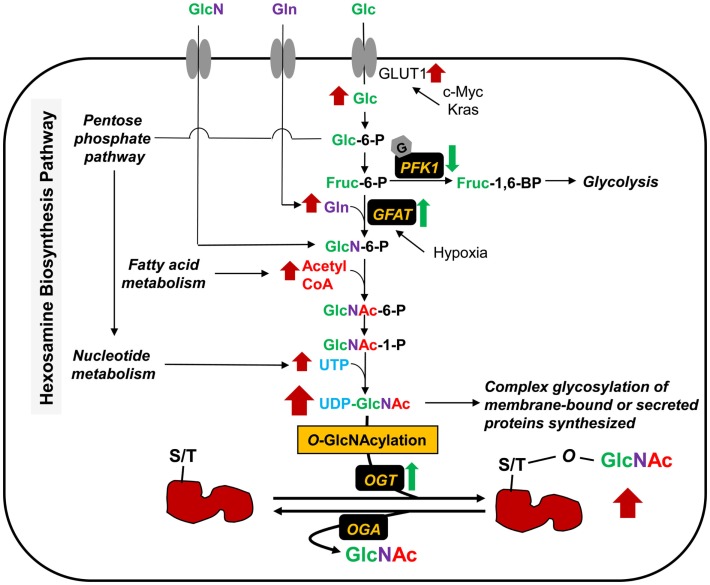
Figure 1.
Metabolic shifts through the hexosamine biosynthesis pathway (HBP) and protein O-GlcNAcylation in cancer. The HBP produces UDP-GlcNAc from its parts, glucose (Glc), glutamine (Gln), acetyl-CoA, and UTP. The levels of these various metabolic inputs are all increased in cancer cells. Glucose is transported into cells by glucose transporters (e.g., GLUT1). Overexpression or mutation of c-Myc and Kras leads to an increase of glucose uptake through GLUT1 activation. O-GlcNAcylation of PFK1 suppresses the enzyme activity, resulting in redirection of glucose metabolism. GFAT is the rate-limiting enzyme for glucose entry into the HBP, which converts Fruc-6-P and glutamine (Gln) into GlcN-6-P. Hypoxia induces the GFAT transcription and expression. Glucosamine (GlcN) enters into cells via the glucose transporters and is phosphorylated to GlcN-6-P by hexokinase, bypassing GFAT. UDP-GlcNAc serves as a sugar donor of classical glycosylation and O-GlcNAcylation. The later glycosylation, taking place in cytoplasm, nucleus, and mitochondria, is controlled by O-GlcNAc cycling enzymes; OGT and OGA for the addition and removal of sugar in and out of proteins, respectively. OGT level is also upregulated, and consequently results in an increase of O-GlcNAcylation in several kinds of cancers.