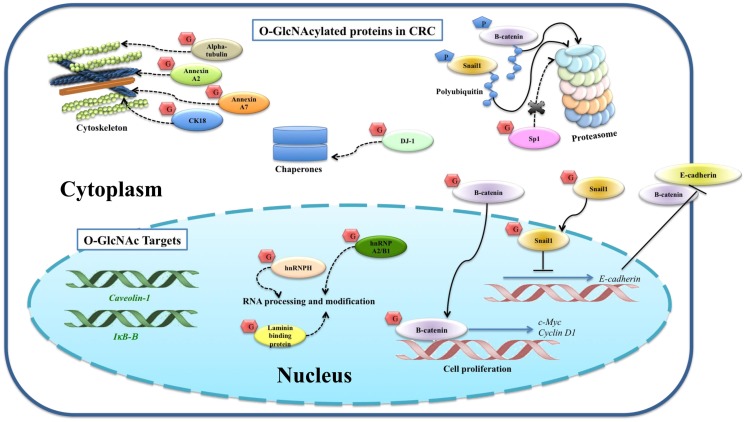
Figure 2.
O-GlcNAcylated proteins and their targets identified in colorectal cancer. O-GlcNAcylation stabilizes β-catenin and subsequently translocates into the nucleus for its gene activation. O-GlcNAcylation stabilizes Snail1, which subsequently represses E-cadherin expression level observed in other cancer cells, suggesting a proposed mechanism for colorectal cancer. Phosphorylation of β-catenin and Snail1 is proposed to activate proteasomal degradation. The proposed mechanisms for other O-GlcNAcylated proteins, including SP1, CK18, α-tubulin, hnRNPA2/B1, hnRNPH, annexin A2, annexin A7, laminin-binding protein, and protein DJ-1, are also indicated. Expression levels of E-cadherin, β-catenin, caveolin-1, and IκB-β are altered corresponding to increased global O-GlcNAcylation, so they are categorized as O-GlcNAc downstream targets. Solid lines indicate known mechanisms, whereas dashed lines are proposed mechanisms.