Abstract
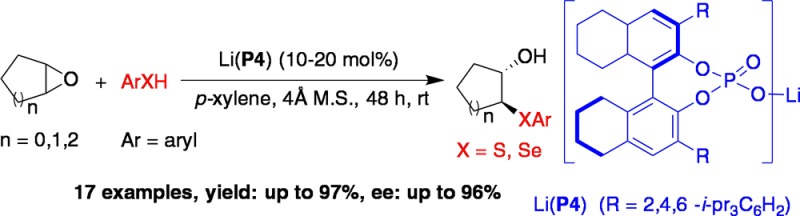
A highly enantioselective method for desymmetrization of meso-epoxides using thiols is reported. This is the first example of epoxide activation achieved using metal BINOL phosphates. The reaction has a broad scope in terms of epoxide substrates and aromatic thiol nucleophiles. The resulting β-hydroxyl sulfides are obtained in excellent yield and enantioselectivity.
The recent finding by Ishihara that a chiral calcium phosphate complex was responsible for high selectivity in the addition of 1,3-dicarbonyl compounds to imines has resulted in considerable interest in chiral biaryl ligated metal phosphates.1 Since then, various alkali and alkaline earth metal complexes of BINOL or VAPOL phosphates have been shown to catalyze a number of reactions involving imines,2 oxindoles,3 trifluoropyruvates,4 β,γ-unsaturated α-ketoesters,5 enamides,6 and enecarbamates.7 Although the metal phosphates have been successful in activating a broad spectrum of functional groups, activation of carbonyl functionality such as aldehydes8 and ketones9 is rare, while epoxide activation using metal phosphates remains an unattained challenge.
Asymmetric nucleophilic ring opening of meso-epoxides is a powerful method to synthesize chiral 1,2- functionalized β-hydroxy compounds.10 Although a wide range of azide,11 amine,12 oxygen,13 and carbon-centered nucleophiles14 have been employed for the desymmetrization of meso-epoxides, the use of thiols remains relatively limited.15 The β-hydroxy sulfide, a synthetic precursor for chiral S,O ligands,16 is also found in many biologically active molecules.17 The potent calcium channel blocker Diltiazem is an example of a β-hydroxy sulfide with only one diastereomer exhibiting the desired biological activity.18
One of the first examples of thiolysis of epoxides with excellent yield and enantioselectivity was reported by Shibasaki15c using a gallium–lithium–bis(binaphthoxide) complex. The scope of the reaction was restricted to only tert-butyl thiol. In another example, a Ga–Ti-salen catalyzed desymmetrization reaction produced the β-hydroxy sulfide with only moderate asymmetric induction, and sub-ambient temperature was required for the observed selectivity.15e Recently, TRIP phosphoric acid catalyzed epoxide ring opening with thiols was reported. This method, though unsuccessful with typical aromatic thiols, was successful with 2-mercaptobenzathiozole as well as its derivatives, and the products were obtained with moderate to good enantioselectivities.19 Overall, the existing catalytic systems are either unsuccessful in attempts to attain superior levels of asymmetric induction or they exhibited limited substrate scope. Therefore, developing a new, versatile catalytic system for such a transformation is highly desired.
In an ongoing effort to attain activation of new electrophiles by phosphate metal complexes, we hypothesized that the phosphate-bound Lewis acidic metal center could activate epoxides toward nucleophilic addition, generating chiral 1,2-functionalized compounds. Herein, we report the first example of highly enantioselective ring opening of meso-epoxides with thiols catalyzed by a Li-BINOL phosphate.
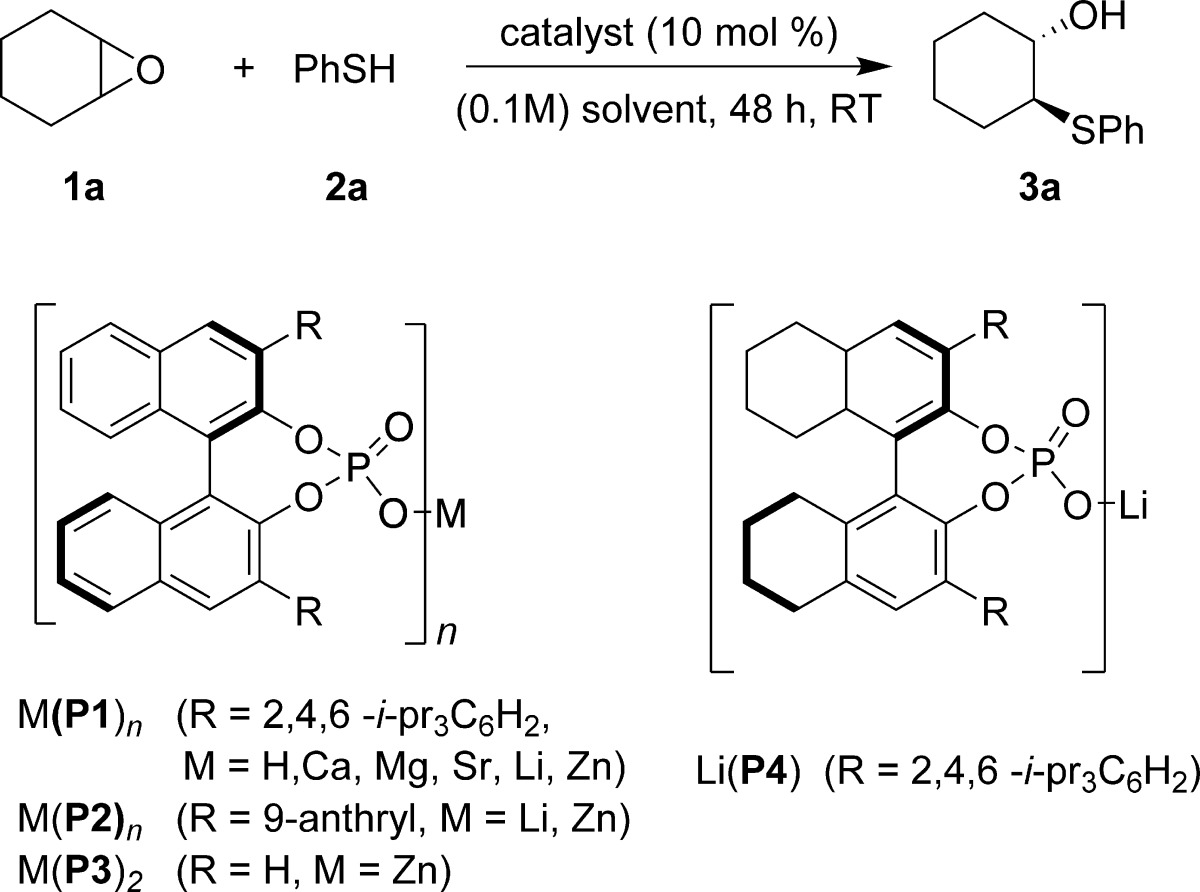
We began our investigation by screening alkali, alkaline earth, and transition metal BINOL phosphates for the desymmetrization of cyclohexene oxide with thiophenol (Table 1). Calcium and magnesium phosphate did not catalyze the desymmetrization reaction (entries 1 and 2). To our delight, Sr-TRIP-BINOL phosphate [Sr(P1)] catalyst allowed us to obtain the desired epoxide ring-opened product, although with no enantioselectivity (entry 3).
Table 1. Reaction Optimization for Catalytic Desymmetrization of Cyclohexene Oxide with Thiophenol.
| entrya | catalyst | solvent | yield (%)b | ee (%)c |
|---|---|---|---|---|
| 1 | Ca(P1) | toluene | 0 | 0 |
| 2 | Mg(P1) | toluene | 0 | 0 |
| 3 | Sr(P1) | toluene | 46 | 0 |
| 4 | Zn(P2)2 | toluene | 80 | 4 |
| 5 | Zn(P1)2 | toluene | 88 | 8 |
| 6 | Zn(P3)2 | toluene | 93 | 60d |
| 7 | Li(P2) | toluene | 78 | 20 |
| 8 | Li(P1) | toluene | 90 | 40 |
| 9 | Li(P1) | toluene | 90 | 60d |
| 10 | Li(P1) | toluene | 92 | 83d,e |
| 11 | Li(P4) | toluene | 93 | 88d,e |
| 12 | Li(P4) | p-xylene | 97 | 91d,e |
| 13 | Li(P4) | p-xylene | 82 | 87e |
| 14 | H(P1) | toluene | 0 | 0 |
| 15 | Li(O-i-Pr) | toluene | 95 | 0 |
Reaction conditions: 1.0 equiv of epoxide and 1.2 equiv of thiol were used.
Isolated yields.
Enantioselectivity was determined using chiral HPLC.
20 mol % catalyst was used.
Using 4 Å MS.
Sterically hindered Zn-BINOL phosphate catalysts [Zn(P1)2 and Zn(P2)2] afforded good yield of the product, albeit with poor asymmetric induction (entries 4 and 5). However, it is worth noting that just Zn-BINOL phosphate [Zn(P3)2], which does not bear any sterically demanding substituents at the 3,3′-positions of the BINOL scaffold, generated the product with 60% ee (entry 6). We observed excellent yield and moderate asymmetric induction with a lithium complex of 9-anthryl and TRIP-BINOL phosphate [Li(P2) and Li(P1)] as the catalyst (entries 7 and 8). Upon increasing the catalyst loading of [Li(P1)] to 20 mol %, a slight enhancement (60% ee) in the asymmetric induction was observed (entry 9). A marked improvement in the enantioselectivity was found when molecular sieves were used, indicating that the residual water in the reaction mixture has a deleterious effect on the enantioselectivity (entry 10). Upon extensive optimization, a lithium complex of H8-TRIP-BINOL phosphate [Li(P4)] and p-xylene was found to be the most suitable catalyst and solvent, respectively (entry 12). Decreasing the catalyst loading to 10 mol % resulted in a minimal drop in yield and enantioselectivity (entry 13). In control experiments, chiral phosphoric acid catalysis did not yield any product (entry 14), while racemic β-hydroxyl sulfide was obtained in excellent yield when Li(O-i-Pr) was used as a catalyst for the ring opening reaction (entry 15).
After establishing the optimal reaction conditions, we explored the use of various epoxides for nucleophilic addition with thiols. The reactions were performed at 10 and 20 mol % of catalyst loading; the results are summarized in Table 2. Epoxides fused to six-membered rings were found to be excellent substrates, resulting in good yields and excellent enantioselectivities (entries 1–3). The reaction of cycloheptene oxide produced the ring-opened product with good asymmetric induction but only in modest yield (entry 4). The use of cyclopentene oxide as a substrate for this reaction obtained the β-hydroxyl sulfide in excellent yield, but only moderate ee was observed (entry 5). We then explored the enantioselective ring opening of both cis- and trans-1,4-cyclohexadiene dioxide. The reaction of trans-1,4-cyclohexadiene dioxide gave 1,3-ring-opened product in 67% yield and with extremely good enantioselectivity when 2 equiv of thiol was used (entry 6). cis-1,4-Cyclohexadiene dioxide achieved excellent yield and ee for the mono-ring-opened product (entry 7). For acyclic epoxide, Li-BINOL phosphate did not yield the desymmetrization product. Unexpectedly, upon further screening, we observed excellent asymmetric induction and yield with a Zn-BINOL phosphate complex for the stilbene oxide desymmetrization with thiophenol (entry 8). To the best of our knowledge, the only other example of Zn-phosphate as a catalyst was reported for an enantioselective cyclopropanation reaction.20
Table 2. Epoxide Substrate Scope for Catalytic Desymmetrization Reaction with Thiophenol.
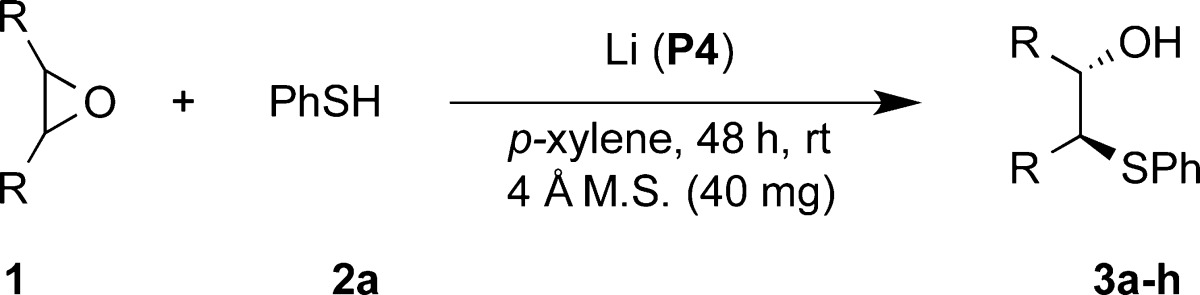
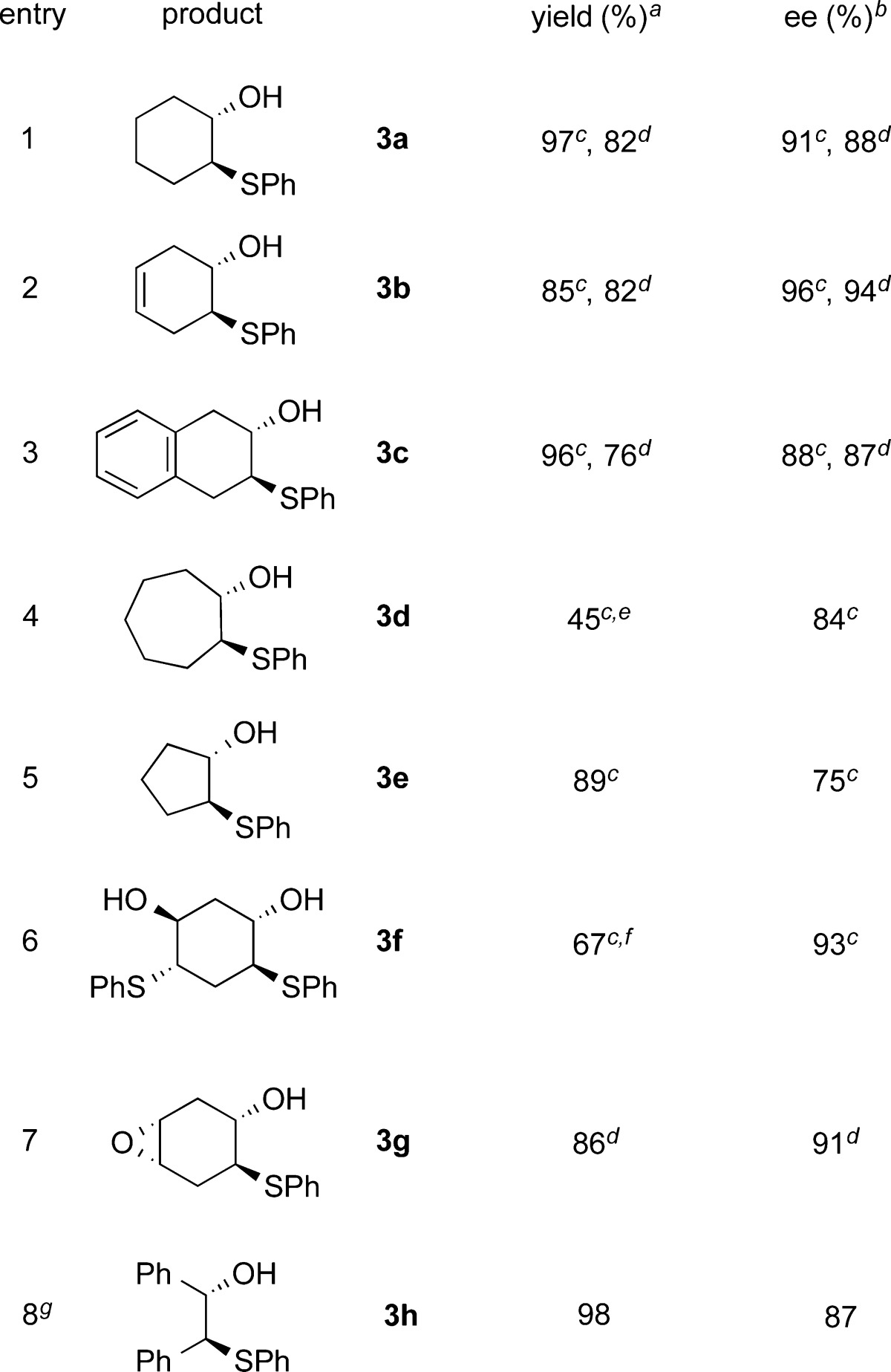
Reaction conditions: 1.0 equiv of epoxide and 1.2 equiv of thiol were used. Isolated yields.
Enantioselectivity was determined using chiral HPLC analysis, and the absolute configuration of the products was confirmed by comparing optical rotations of compounds 3a and 3h with the reported literature values, and the configuration of the other compounds was analogously assigned.15l
20 mol % catalyst was used.
10 mol % catalyst was used.
Reaction stirred for 96 h at rt.
2.2 equiv of thiol was used.
Reaction conditions: Zn(P3)2 (10 mol %), toluene, 48 h, rt.
Next, we explored the scope of thiol nucleophiles for the epoxide ring opening reaction. The reaction worked well with 10 mol % catalyst loading. The results are summarized in Table 3. Aromatic thiols with electron-withdrawing groups produced β-hydroxy sulfides in excellent yield and asymmetric induction (4b and 4c). Thiols with electron-donating substituents were also well-tolerated, generating the ring-opened product with excellent yield and enantioselectivity (4d–4g). Bulky thiols, such as 2-naphthylthiol, were also found to be excellent substrates, affording the product with 94% ee (4h). We also examined thiols with heteroatom-containing functional groups. 2-Amino thiophenol as a substrate resulted in exclusively the β-hydroxy sulfide product with excellent yield and asymmetric induction (4i). Also, the reaction employing methyl thiosalicylate yielded the epoxide desymmetrization product in 96% ee (4j). These results indicate that heteroatom-containing thiols are compatible with the lithium phosphate catalytic system.
Table 3. Catalytic Ring Opening of 4,5-Epoxycyclohexene with Thiols.
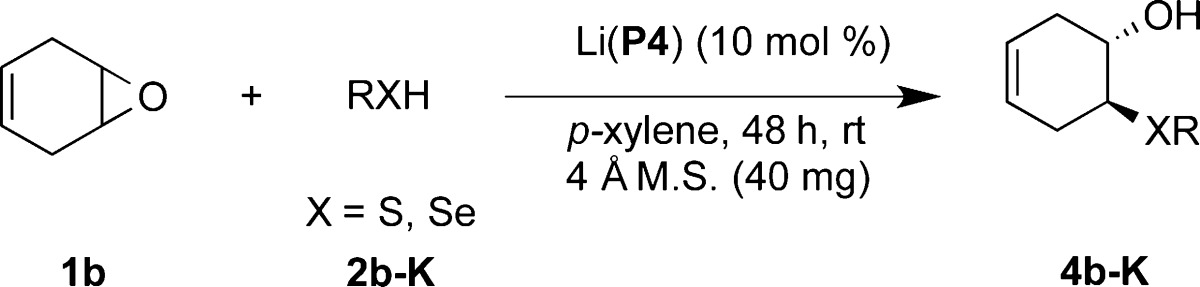
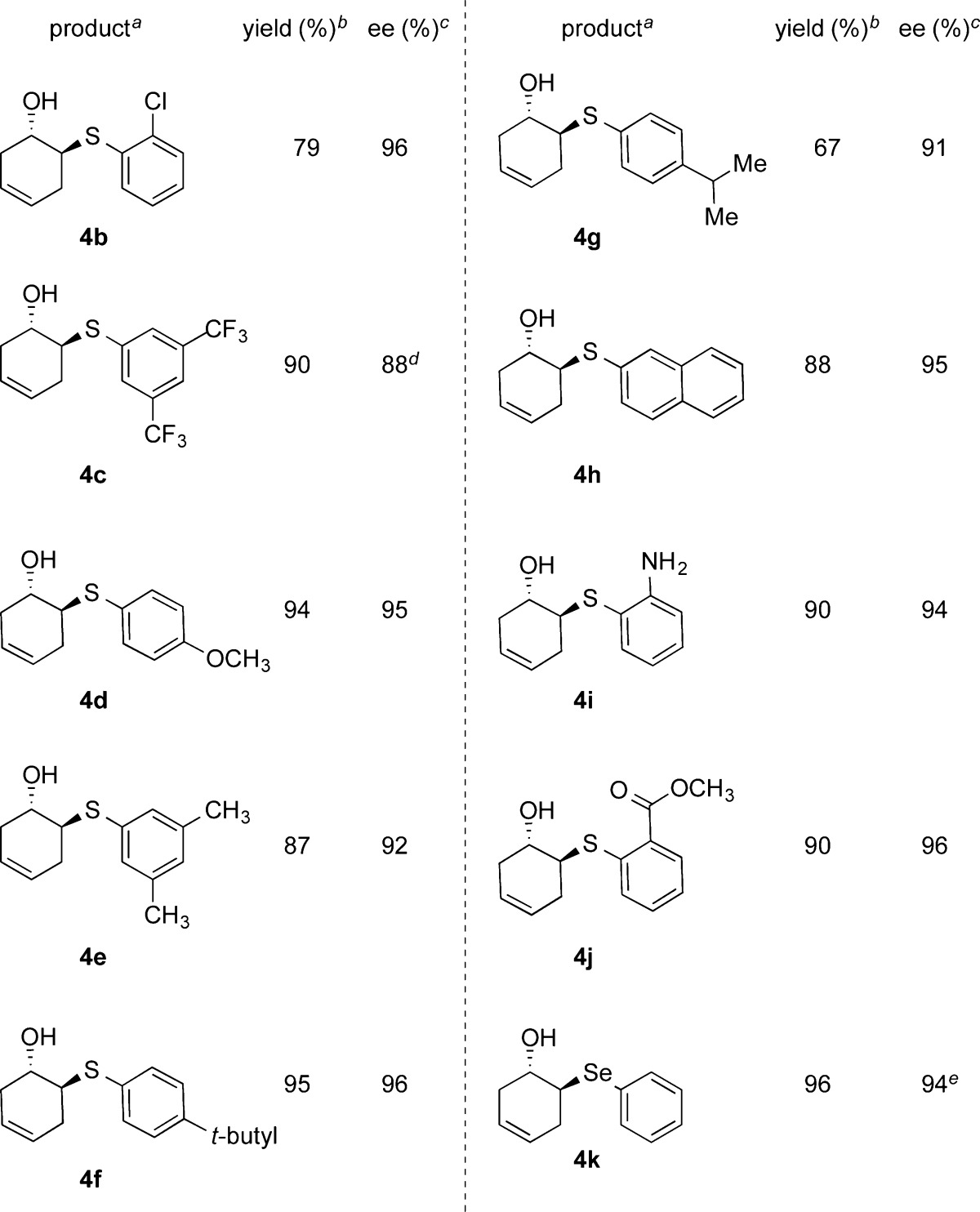
Reaction conditions: 1.0 equiv of epoxide and 1.2 equiv of thiol were used.
Isolated yields.
Enantioselectivity was determined using chiral HPLC, and the absolute configuration of the compounds in this table was analogously assigned (for more information, see footnote of Table 2).
Product was protected with the 3,4-dinitrobenzoyl group for chiral HPLC analysis.
Reaction time: 24 h.
Chiral organo-selenium compounds are useful intermediates in many asymmetric transformations.15l,21 Fortuitously, we were able to extend the lithium phosphate catalyzed epoxide ring opening methodology to a selenophenol nucleophile, resulting in a chiral β-arylseleno alcohol in excellent yield and 94% ee at ambient temperature (4k). It is worth mentioning that the other methods reported in the literature required lower reaction temperature to achieve similar levels of asymmetric induction.15l,21 The absolute configuration of the products was confirmed by comparing optical rotations of compounds 3a and 3h with the reported literature values,15l and the configuration of the other compounds was analogously assigned.
Desymmetrization of meso-epoxide catalyzed by Li-BINOL phosphates has a wide substrate scope in terms of aromatic thiols. Regrettably, when aliphatic thiols were employed, we observed little to no product formation. We hypothesize that the phosphate-bound lithium metal acts as a Lewis acid, activating the epoxide, and the phosphoryl moiety behaves as a Brønsted basic site, activating the thiophenol. We reason that, because aromatic thiols have lower pKa values relative to that of aliphatic thiols, the phosphoryl oxygen moiety of the Li-BINOL phosphate is better able to activate aromatic thiols for the epoxide ring opening reaction (Figure 1).
Figure 1.
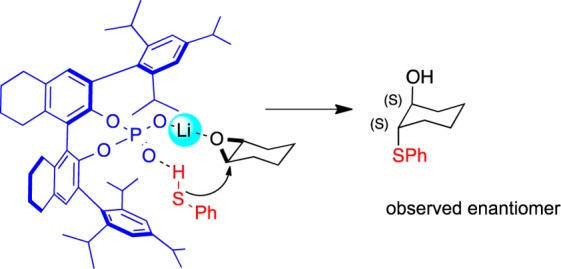
Proposed TS for desymmetrization of meso-epoxides with thiols catalyzed by lithium BINOL phosphate.
Overall, we have developed a highly enantioselective method for desymmetrization of meso-epoxides using thiols. This is the first example of epoxide activation achieved using a BINOL phosphate metal complex. The reaction has a broad substrate scope in terms of epoxides and also is successful with a wide range of aromatic thiols. This new catalytic activation of epoxides has features superior to existing options in terms of selectivity, being able to achieve higher levels of asymmetric induction in the ring-opened products. This new activation protocol could potentially be used for the desymmetrization of epoxides with an even broader scope of nucleophiles such as azides and amines, as well as for kinetic resolution studies.
Acknowledgments
We thank the National Institutes of Health (NIH GM-082935) and the National Science Foundation CAREER program (NSF-847108) for financial support.
Supporting Information Available
Experimental details and characterization with spectral data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- a Hatano M.; Moriyama K.; Maki T.; Ishihara K. Angew. Chem., Int. Ed. 2010, 49, 3823. [DOI] [PubMed] [Google Scholar]; b Klussmann M.; Ratjen L.; Hoffmann S.; Wakchaure V.; Goddard R.; List B. Synlett 2010, 2189. [Google Scholar]
- a Ingle G. K.; Liang Y.; Mormino M. G.; Li G.; Fronczek F. R.; Antilla J. C. Org. Lett. 2011, 13, 2054. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Larson S. E.; Li G.; Rowland G. B.; Junge D.; Huang R.; Woodcock H. L.; Antilla J. C. Org. Lett. 2011, 13, 2188. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Shi S.-H.; Huang F.-P.; Zhu P.; Dong Z.-W.; Hui X.-P. Org. Lett. 2012, 14, 2010. [DOI] [PubMed] [Google Scholar]; d Nimmagadda S. K.; Zhang Z.; Antilla J. C. Org. Lett. 2014, 16, 4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Zheng W.; Zhang Z.; Kaplan M. J.; Antilla J. C. J. Am. Chem. Soc. 2011, 133, 3339. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zhang Z.; Zheng W.; Antilla J. C. Angew. Chem., Int. Ed. 2011, 50, 1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueping M.; Bootwicha T.; Kambutong S.; Sugiono E. Chem.—Asian J. 2012, 7, 1195. [DOI] [PubMed] [Google Scholar]
- Lv J.; Li X.; Zhong L.; Luo S.; Cheng J.-P. Org. Lett. 2010, 12, 1096. [DOI] [PubMed] [Google Scholar]
- Drouet F.; Lalli C.; Liu H.; Masson G.; Zhu J. Org. Lett. 2011, 13, 94. [DOI] [PubMed] [Google Scholar]
- Alix A.; Lalli C.; Retailleau P.; Masson G. J. Am. Chem. Soc. 2012, 134, 10389. [DOI] [PubMed] [Google Scholar]
- a Hanamoto T.; Furuno H.; Sugimoto Y.; Inanaga J. Synlett 1997, 1, 79. [Google Scholar]; b Hayano T.; Sakaguchi T.; Furuno H.; Ohba M.; Okawa H.; Inanaga J. Chem. Lett. 2003, 32, 608. [Google Scholar]; c Terada M.; Soga K.; Momiyama N. Angew. Chem., Int. Ed. 2008, 47, 4122. [DOI] [PubMed] [Google Scholar]; d Momiyama N.; Tabuse H.; Terada M. J. Am. Chem. Soc. 2009, 131, 12882. [DOI] [PubMed] [Google Scholar]; e Jain P.; Antilla J. C. J. Am. Chem. Soc. 2010, 132, 11884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Hatano M.; Ikeno T.; Matsumura T.; Torii S.; Ishihara K. Adv. Synth. Catal. 2008, 350, 1776. [Google Scholar]; b Zhang Z.; Jain P.; Antilla J. C. Angew. Chem., Int. Ed. 2011, 50, 10961. [DOI] [PubMed] [Google Scholar]; c Zhang Z.; Antilla J. C. Angew. Chem., Int. Ed. 2012, 51, 11778. [DOI] [PubMed] [Google Scholar]
- a Nielsen L. P. C.; Jacobsen E. N.. Aziridines and Epoxides in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2006; p 229. [Google Scholar]; b Pineschi M. Eur. J. Org. Chem. 2006, 4979. [Google Scholar]
- a Martinez L. E.; Leighton J. L.; Carsten D. H.; Jacobsen E. N. J. Am. Chem. Soc. 1995, 117, 5897. [Google Scholar]; b Leighton J. L.; Jacobsen E. N. J. Org. Chem. 1996, 61, 389. [Google Scholar]; c Jacobsen E. N. Acc. Chem. Res. 2000, 33, 421. [DOI] [PubMed] [Google Scholar]
- a Sekine A.; Ohshima T.; Shibasaki M. Tetrahedron 2002, 58, 75. [Google Scholar]; b Bartoli G.; Bosco M.; Carlone A.; Locatelli M.; Massaccesi M.; Melchiorre P.; Sambri L. Org. Lett. 2004, 6, 2173. [DOI] [PubMed] [Google Scholar]; c Arai K.; Salter M. M.; Yamashita Y.; Kobayashi S. Angew. Chem. 2007, 46, 955. [DOI] [PubMed] [Google Scholar]; d Gao B.; Wen Y.; Yang Z.; Huang X.; Liu X.; Feng X. Adv. Synth. Catal. 2008, 350, 385. [Google Scholar]
- a Tokunaga M.; Larrow J. F.; Kakiuchi F.; Jacobsen E. N. Science 1997, 277, 936. [DOI] [PubMed] [Google Scholar]; b Jacobsen E. N.; Kakiuchi F.; Konsler R. G.; Larrow J. F.; Tokunaga M. Tetrahedron Lett. 1997, 38, 773. [Google Scholar]; c Nozaki K.; Nakano K.; Hiyama T. J. Am. Chem. Soc. 1999, 121, 11008. [DOI] [PubMed] [Google Scholar]; d Matsunaga S.; Das J.; Roels J.; Vogl E. M.; Yamamoto N.; Iida T.; Yamaguchi K.; Shibasaki M. J. Am. Chem. Soc. 2000, 122, 2252. [Google Scholar]
- a Shimizu K. D.; Cole M. B.; Krueger C. A.; Kuntz K. W.; Snapper M. L.; Hoveyda A. H. Angew. Chem., Int. Ed. Engl. 1997, 36, 1704. [Google Scholar]; b Crotti P.; Di Bussolo V.; Favero L.; Macchia F.; Pineschi M. Gazz. Chim Acta 1997, 127, 273. [Google Scholar]; c Oguni N.; Miyagi Y.; Itoh K. Tetrahedron Lett. 1998, 39, 9023. [Google Scholar]; d Schaus S. E.; Jacobsen E. N. Org. Lett. 2000, 2, 1001. [DOI] [PubMed] [Google Scholar]; e Bandini M.; Cozzi P. G.; Melchiorre P.; Umani-Ronchi A. Angew. Chem., Int. Ed. 2004, 43, 84. [DOI] [PubMed] [Google Scholar]; f Belokon Y.N.; Chusov D.; Peregudov A. S.; Yashkina L. V.; Timofeeva G. I.; Maleev V. I.; North M.; Kagan H. B. Adv. Synth. Catal. 2009, 351, 3157. [Google Scholar]
- a Yamashita H.; Mukaiyama T. Chem. Lett. 1985, 14, 1643. [Google Scholar]; b Yamashita H. Bull. Chem. Soc. Jpn. 1988, 61, 1213. [Google Scholar]; c Iida T.; Yamamoto N.; Sasai H.; Shibasaki M. J. Am. Chem. Soc. 1997, 119, 4783. [Google Scholar]; d Wu M. H.; Jacobsen E. N. J. Org. Chem. 1998, 63, 5252. [DOI] [PubMed] [Google Scholar]; e Wu J.; Hou X. L.; Dai L. X.; Xia L. J.; Tang M. H. Tetrahedron: Asymmetry 1998, 9, 3431. [Google Scholar]; f Boudou M.; Ogawa C.; Kobayashia S. Adv. Synth. Catal. 2006, 348, 2585. [Google Scholar]; g Zhou Z.; Li Z.; Quanyong W.; Liu B.; Li K.; Zhao G.; Zhou Q.; Tang C. J. Organomet. Chem. 2006, 691, 5790. [Google Scholar]; h Nandakumar M. V.; Tschöp A.; Krautscheid H.; Schneider C. Chem. Commun. 2007, 275. [DOI] [PubMed] [Google Scholar]; i Chen Y.-J.; Chen C. Tetrahedron: Asymmetry 2007, 18, 1313. [Google Scholar]; j Tschöp A.; Nandakumar M. V.; Pavlyuk O.; Schneider C. Tetrahedron Lett. 2008, 49, 1030. [Google Scholar]; k Sun J.; Yuan F.; Yang M.; Pan Y.; Zhu C. Tetrahedron Lett. 2009, 50, 548. [Google Scholar]; l Sun J.; Yang M.; Yuan F.; Jia X.; Yang X.; Pan Y.; Zhu C. Adv. Synth. Catal. 2009, 351, 920. [Google Scholar]; m Nandakumar M. V.; Ghosh S.; Schneider C. Eur. J. Org. Chem. 2009, 6393. [Google Scholar]
- Mellah M.; Voituriez A.; Schulz E. Chem. Rev. 2007, 107, 5133. [DOI] [PubMed] [Google Scholar]
- a Wipf P.; Jeger P.; Kim Y. Bioorg. Med. Chem. Lett. 1998, 8, 351. [DOI] [PubMed] [Google Scholar]; b Martinelli M. J.; Vaidyanathan R.; Khau V. V.; Staszak M. A. Tetrahedron Lett. 2002, 43, 3365. [Google Scholar]
- a Schwartz A.; Madan P. B.; Mohacsi E.; O’Brien J. P.; Todaro L. J.; Coffen D. L. J. Org. Chem. 1992, 57, 851. [Google Scholar]; b Yamada S.-i.; Mori Y.; Morimatsu K.; Ishizu Y.; Ozaki Y.; Yoshioka R.; Nakatani T.; Seko H. J. Org. Chem. 1996, 61, 8586. [Google Scholar]
- Wang Z.; Law W. K.; Sun J. Org. Lett. 2013, 15, 5964. [DOI] [PubMed] [Google Scholar]
- Lacasse M.-C.; Poulard C.; Charette A. B. J. Am. Chem. Soc. 2005, 127, 12440. [DOI] [PubMed] [Google Scholar]
- a Tiecco M.; Testaferri L.; Santi C.; Tomassini C.; Bonini R.; Marini F.; Bagnoli L.; Temperini A. Org. Lett. 2004, 6, 4751. [DOI] [PubMed] [Google Scholar]; b Okamoto K.; Nishibayashi Y.; Uemura S.; Toshimitsu A. Tetrahedron Lett. 2004, 45, 6137. [Google Scholar]; c Yang M.; Zhu C.; Yuan F.; Huang Y.; Pan Y. Org. Lett. 2005, 7, 1927. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


