Table 1. Reaction Optimization for Catalytic Desymmetrization of Cyclohexene Oxide with Thiophenol.
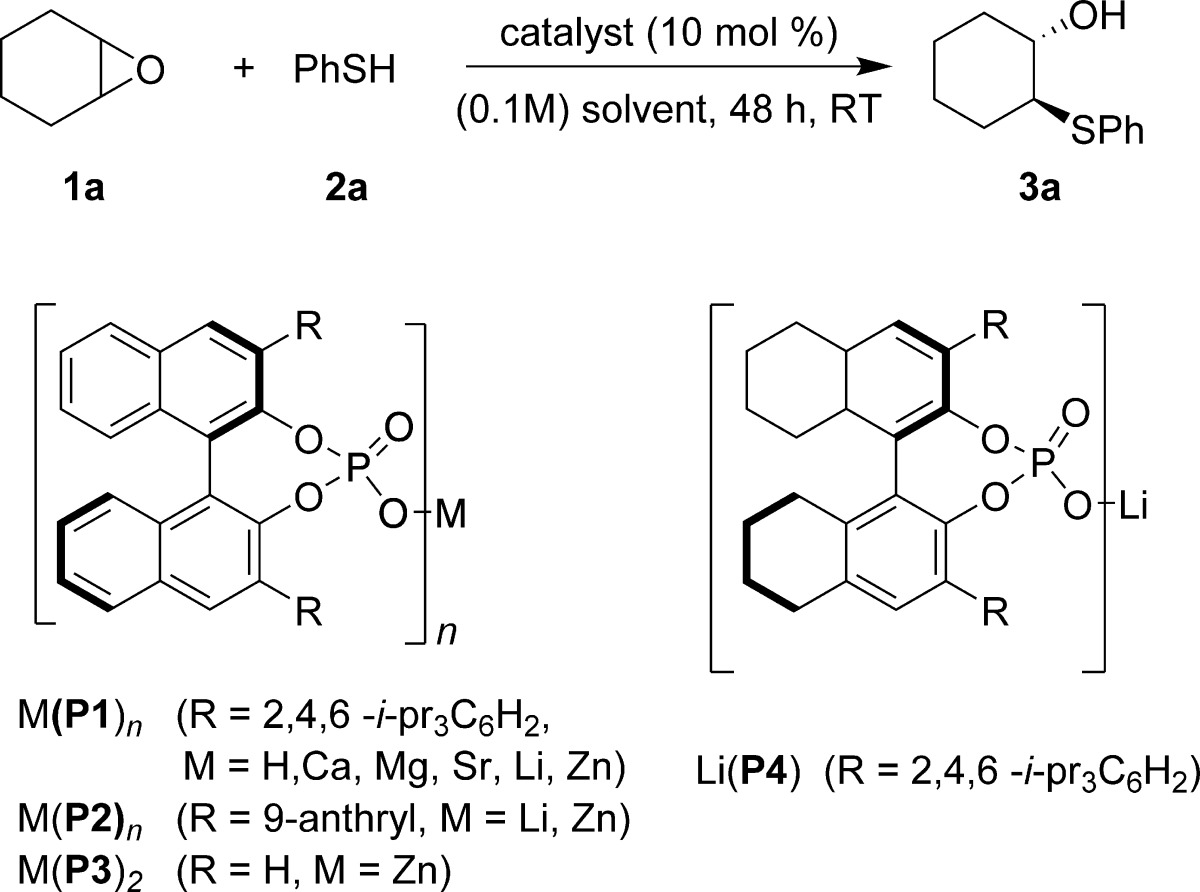
| entrya | catalyst | solvent | yield (%)b | ee (%)c |
|---|---|---|---|---|
| 1 | Ca(P1) | toluene | 0 | 0 |
| 2 | Mg(P1) | toluene | 0 | 0 |
| 3 | Sr(P1) | toluene | 46 | 0 |
| 4 | Zn(P2)2 | toluene | 80 | 4 |
| 5 | Zn(P1)2 | toluene | 88 | 8 |
| 6 | Zn(P3)2 | toluene | 93 | 60d |
| 7 | Li(P2) | toluene | 78 | 20 |
| 8 | Li(P1) | toluene | 90 | 40 |
| 9 | Li(P1) | toluene | 90 | 60d |
| 10 | Li(P1) | toluene | 92 | 83d,e |
| 11 | Li(P4) | toluene | 93 | 88d,e |
| 12 | Li(P4) | p-xylene | 97 | 91d,e |
| 13 | Li(P4) | p-xylene | 82 | 87e |
| 14 | H(P1) | toluene | 0 | 0 |
| 15 | Li(O-i-Pr) | toluene | 95 | 0 |
Reaction conditions: 1.0 equiv of epoxide and 1.2 equiv of thiol were used.
Isolated yields.
Enantioselectivity was determined using chiral HPLC.
20 mol % catalyst was used.
Using 4 Å MS.
