Abstract
STUDY QUESTION
Does laser-assisted zona thinning of cleavage stage mouse embryos facilitate hatching in vitro?
SUMMARY ANSWER
No, unlike laser zona opening, zona thinning does not facilitate embryo hatching.
WHAT IS KNOWN ALREADY
Artificial opening of the zona pellucida facilitates hatching of mouse and human embryos. Laser-assisted zona thinning has also been used for the purpose of assisted hatching of human embryos but it has not been properly investigated in an animal model; thinning methods have produced inconsistent clinical results.
STUDY DESIGN, SIZE, DURATION
Time-lapse microscopy was used to study the hatching process in the mouse after zona opening and zona thinning; a control group of embryos was not zona-manipulated but exposed to the same laser energy.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Eight-cell CB6F1/J mouse embryos were pooled and allocated to three groups (n = 56 per group): A control group of embryos that were exposed to a dose of laser energy focused outside the zona pellucida (zona intact); one experimental group of embryos in which the zona pellucida was opened by complete ablation using the same total number of pulses as the control group; a second experimental group of embryos in which the zona pellucida was thinned to establish a smooth lased area using the same number of pulses as used in the other two groups. The width of the zona opening was 25 μm and width of the thinned area was 35 μm. Development was monitored by time-lapse microscopy. Overall treatment differences for continuous variables were analyzed by analysis of variance and pairwise comparisons using the Student t-test allowing for unequal variances, while for categorical data, a standard chi-squared test was utilized for all pairwise comparisons.
MAIN RESULTS AND THE ROLE OF CHANCE
The frequency of complete hatching was 33.9% in the control group, 94.4% after zona opening, and 39.3% after zona thinning (overall group comparison, P < 0.0001). Overall, 60.7% of the zona-thinned embryos did not complete the hatching process and remained trapped within the zona; when they did hatch, they did not necessarily hatch from the zona-thinned area. Hatching in about one-third of the zona-intact embryos began with breaches at multiple sites by small groups of cells. Likewise, 53.6% of zona-thinned embryos had multiple breaches, always involving an area outside the thinned zone. Zona opening decreased multiple breaching and led to blastocyst escape an average of 14 h earlier than zona-thinned embryos and 5.5 h before control embryos (P = 0.0003).
LIMITATIONS, REASONS FOR CAUTION
The experiments presented here were limited to in vitro experiments performed in the mouse. Whether human embryos would behave the same way under similar circumstances is unknown. We postulate that zona thinning is not beneficial in human embryos.
WIDER IMPLICATIONS OF THE FINDINGS
The experiments demonstrate that zona thinning is not equivalent to zona opening for assisted hatching. The study provides reason for systematic reviews of assisted hatching trials to take the method of assisted hatching into consideration and not combine the results of zona thinning and zona opening procedures.
STUDY FUNDING/COMPETING INTEREST(S)
Institutional funds were used for the study. No competing interests are declared.
Keywords: alternate hatching, assisted hatching, multiple breaching, zona pellucida opening
Introduction
Hatching or escape from the zona pellucida is a prerequisite for mammalian embryo implantation (McLaren, 1969). During development in vivo, hatching is initiated and completed under the influence and control of both the embryo and the reproductive tract. In a number of species including mouse, bovine and the human, hatching is also supported by modern in vitro culture conditions, but the frequency, timing and mechanism of the process may be altered or negatively influenced (Edwards et al., 1986).
In the early days of human IVF, it was noted that only a small proportion of expanded human blastocysts underwent spontaneous hatching in vitro following extended culture (Cohen et al., 1990). This was attributed to in vitro culture itself but also to embryo quality, which in turn could reflect patient characteristics (Cohen et al., 1992). At the same time, experiments in the mouse showed that hatching occurred earlier and more frequently after artificially opening the zona pellucida by so-called ‘zona drilling’ (originally proposed for assisted fertilization) (Depypere et al., 1988) or to assist hatching (Malter and Cohen, 1989). Those findings formed the basis for the application of zona drilling to spare human embryos in order to potentially facilitate hatching. Indeed, when applied to spare human embryos, it was observed that they consistently and preferentially hatched through the artificial hole (Cohen et al., 1990). Moreover, much like the mouse model, hatching occurred earlier and more frequently in manipulated human embryos in comparison to non-manipulated embryos (Cohen et al., 1990).
The procedure was thus termed ‘assisted hatching’ and applied clinically during two randomized clinical trials, which demonstrated that assisted hatching was moderately effective in patients with poor prognosis (Cohen et al., 1992). The same study also showed that the procedure was not effective in good prognosis patients.
Following the success of the first clinical trials, it was suggested that the hatching process may also be enhanced by artificially reducing the thickness of the mouse outer zona pellucida (so-called zona thinning) while keeping the inner zona pellucida intact (Khalifa et al., 1992). Although zona thinning appeared to improve hatching in the mouse, the procedure was not beneficial in the human (Tucker et al., 1993). Nonetheless, another clinical investigation of zona thinning was conducted and showed that implantation improved following transfer of zona-thinned embryos in patients with repeated IVF failure (Antinori et al., 1996); this significant technical variation on assisted hatching was thus adopted for clinical use. In addition, as laser technologies became increasingly common for zona manipulation (Das et al., 2009), laser zona thinning with wide variation in the methodology itself also became common. Ultimately, however, there has been no confirmation of the assumption that thinning the zona is equivalent to opening the zona in facilitating hatching (Cohen and Alikani, 2013).
In this study, we used time-lapse microscopy (TLM) to study and compare the dynamics of the hatching process in zona-intact, laser zona-opened and laser zona-thinned mouse embryos in order to investigate whether zona-thinning facilitates hatching.
Materials and Methods
CB6F1/J female mice (The Jackson Laboratory, Bar Harbor, ME, USA) younger than 20 weeks were super-ovulated by an i.p. injection of 7.5–10 iu pregnant mare serum gonadotrophin (PMSG, Sigma-Aldrich, St Louis, MO, USA) followed by 7.5–10 iu hCG 47 h later (Sigma-Aldrich) and mated with fertile male CB6F1/J mice.
All culture was performed in Modified KSOMaa medium (Global media – Lifeglobal – IVFonline, Guelph, Ontario, Canada) with human serum albumin (HSA) protein supplement (Sage, In Vitro Fertilization, Inc., Trumbull, CT, USA). One-cell embryos were recovered 21 h post hCG injection from the ampullary region of the oviduct, in 0.03% hyaluronidase in Global-Hepes medium supplemented with 5% (5 mg/ml) HSA. The embryos were rinsed in Global/Hepes/1% (1 mg/ml) HSA at room temperature (22–23°C) and cultured in Global/1% HSA. Culture to the eight cell stage was performed in 100 μl drops under washed and pre-gassed mineral oil (Sigma-Aldrich) in an atmosphere of 5.5% CO2, 5% O2, and balance N2, pH = 7.32 at 37°C, using a Sanyo MCO-5M incubator (Sanyo Biomedical/Panasonic Healthcare, Secaucus, NJ, USA).
At the 8-cell stage of development, embryos were pooled and allocated into three groups by defocusing the microscope in order to prevent any bias in selection. The experiment was repeated five times. The total number of embryos in each group was 56.
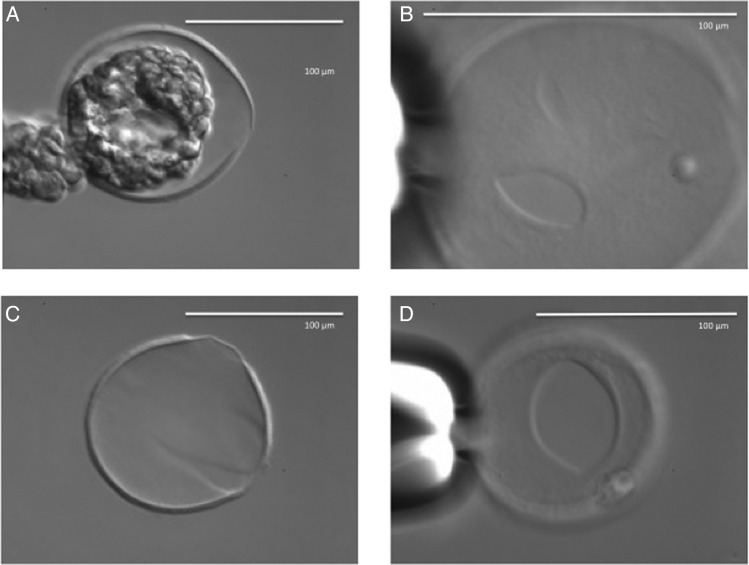
The laser system included the Fertilase 1.48 μm laser, 100 mW, operated by Octax Eyeware digital interface (MTG, Bruckberg, Germany) positioned on an Olympus IX70 inverted microscope equipped with an Octax Adaptive Electronic Condenser (MTG) and Narishige micromanipulators (Narishige, East Meadow, NY, USA). The amount of total laser energy used was the same in all groups during each experiment. Before each experiment, the system was extensively calibrated and lasing was validated in spare embryos in order to standardize the diameter of the ablated area for each experiment. This standard was maintained throughout (Fig. 1).
Figure 1.
Calibration of zona opening (A–C) and thinning (D–F) in the mouse. Same number of pulses were used in each procedure. A calibration pulse was used visible at 4 o'clock in each instance. Experimental embryos were lased after calibration on each day. The calibration pulse was only used in spare embryos. Zona opening consisted of a set row of pulses on the outside of the zona pellucida (A) followed by a row of pulses carefully placed on the inside. The first of those pulses is shown in (B). The scale bar in (A) applies also to (B)–(F).
The control arm of the experiment consisted of embryos that were exposed to a dose of laser energy focused outside the zona pellucida at a constant distance of 10–15 μm from the outer zona limit. The laser dose was the same as that used in the two experimental arms. These ‘sham-lased’ embryos remained zona-intact and are referred to as the control zona-intact group (n = 56).
In the first experimental group of embryos, the zona was opened (classical assisted hatching methodology), by ablating the outside first and the inside last, using the same total number of pulses of 8 × 2.6 ms. The position of each released pulse was carefully controlled. Embryos in which treatment did not conform to the protocol were discarded.
In the second experimental group, the zona was thinned using the same number of pulses as used in the zona-opened and control groups (Fig. 1). The thinning procedure was standardized during pilot experiments to establish a smooth lased area. In a series of other pilot experiments, zona thinning was performed mimicking the principle method used in the human (Antinori et al., 1996; Hiraoka et al., 2009), that is, ablation of one quarter or one half of the zona pellucida circumference. The design of the current zona thinning procedure was based on the outcome of those pilot studies. Clinically, more laser energy is used for zona thinning than for zona opening. The potential effect of a difference in total laser energy between the two methods was removed by using the same amount of laser energy in the current experiments for all groups.
Each embryo in the experimental arms was checked to confirm that the zona was consistently opened fully (assisted hatching) or only sheared on the outside (zona thinning) using the same dimensions established for each protocol. The width of the zona opening was 25 μm. The width of the thinned area was 35 μm. All manipulations were performed at room temperature (22–23°C).
Development was monitored by TLM using an EmbryoScope (Unisense Fertilitech A/S, Aarhus N, Denmark). All embryos were loaded in EmbryoSlide culture dishes with 12 wells (Unisense FertiliTech, Aarhus N, Denmark). EmbryoSlides were prepared the day before allocation of embryos and equilibrated overnight in a Sanyo incubator in an atmosphere of 5.5% CO2, 5.0% O2 and balance N2 at 37°C. Individual wells in the EmbryoSlide contained 25 μl Global/1% HSA covered with 1.5 ml mineral oil (Sigma-Aldrich) for all embryos and experiments. The HSA concentration was shown to produce optimal cell counts at the blastocyst stage (Schimmel and Cohen, unpublished data).
Each slide replicate was loaded with four embryos from each experimental group (one embryo per well). The time-lapse mechanism was turned on immediately after slide loading, and was set to capture one image in one focal plane every 5 min for ∼93 h. Multiple focal images were unnecessary due to the small diameter of the mouse embryo. A single focal plane was chosen for the shortest cycle interval to capture the maximum number of time-lapse images.
Analysis of the timing and mechanism of hatching was performed using the EmbryoViewer workstation after all experiments were completed. A total of 168 embryos were treated and observed over time (56 embryos allocated to each group). The EmbryoViewer system stores all the TLM recordings and allows observers to study timing and morphology of developmental events. As a single reference point is needed for the exact timing of events, the injection of hCG to trigger ovulation was used as the start time for the observations.
Statistical evaluations
All data were reported by means of summary descriptive statistics e.g. mean, SD, minimum, maximum, frequencies and percentages. Hatching rate was the primary outcome measure. Overall treatment differences for variables of a continuous nature (e.g. time to initial breach and complete hatching, overall and in relation to hatching configuration) were analyzed using an ANOVA model. Subsequent pairwise comparisons of the groups for these parameters were carried out using the conservative method of a Student t-test allowing for unequal variances. For the purpose of analysis of categorical data (e.g. hatching rate, hatching configuration), a standard chi-squared test with the appropriate degrees of freedom was utilized for overall and for all pairwise comparisons. In addition, for hatching rates, the relative risk (RR) was calculated for each pairwise comparison. All statistical tests were carried out at the 5% level of significance. The statistical software package was StatPlus:mac for Apple (AnalystSoft, Inc., Alexandria, VA, USA).
Results
Incidence of hatching
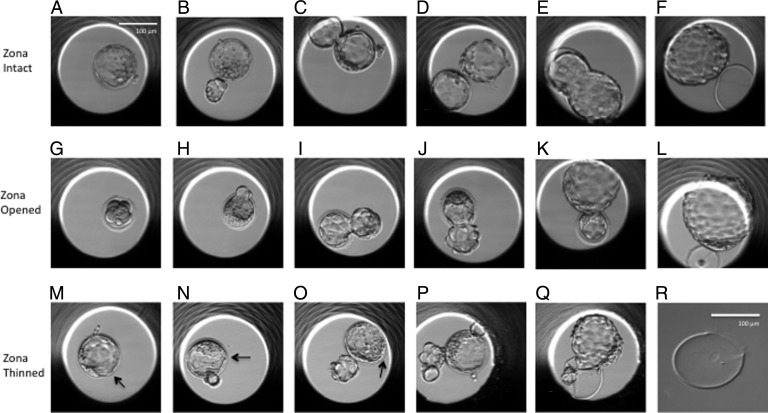
All 168 eight-cell embryos developed to blastocysts with a recognizable blastocoel and inner cell mass (ICM) (Table I). The process of hatching was analyzed in each embryo until final collapse of that embryo. The first breaching of the zona pellucida by zona-breaker cells was seen in 100% of both the zona-intact control embryos and the zona-thinned embryos (Fig. 2). In 2 out of the 56 blastocysts in the zona-opened group, cells did not extrude through the laser hole and the embryos remained within the zona pellucida. The frequency of complete and partial hatching for all groups is presented in Table I. Significantly more zona-opened embryos hatched completely compared with both the zona-intact control embryos (P < 0.0001; RR, 2.78; 95% confidence interval (CI), 1.92–4.03) and the zona-thinned embryos (P < 0.0001; RR, 2.40; 95% CI, 1.72–3.35). Furthermore, with respect to complete hatching, no significant difference was found between the zona-intact control group and the zona-thinned group (P = 0.4871; RR, 1.16; 95% CI, 0.71–1.89).
Table I.
Incidence of partial and complete hatching assessed by time-lapse microscopy (TLM) in zona intact (ZI) control embryos and after zona opening (ZO) by laser assisted hatching or laser zona thinning (ZT) at the 8-cell stage in the mouse.
| Group | 8-cell mouse embryos (N) |
Blastocyst formation | Arrested after cavitation | Fully hatched N (%) |
Partially hatched N (%) |
|---|---|---|---|---|---|
| Control zona-intact (ZI) | 56 | 56 | 0 | 19 (33.9) | 37 (66.1) |
| Laser zona-opened (ZO) | 56 | 56 | 2 | 51 (94.4) | 3 (5.6) |
| Laser zona-thinned (ZT) | 56 | 56 | 0 | 22 (39.3) | 34 (60.7) |
Comparison of fully hatched versus partially hatched:
Overall group comparison using analysis of variance (ANOVA): P < 0.0001.
Pairwise comparisons using T-test
ZI versus ZO: P < 0.0001.
ZI versus ZT: NS.
ZO versus ZT: P < 0.0001.
Figure 2.
Mouse blastocysts from time-lapse studies. Embryos in the top row (A–F) are zona-intact control embryos. Three groups of breaker cells are visible in the embryo in (A) at 1, 2 and 4 o'clock positions. The embryo in (B) is hatching, but a second group of breaker cells is seen at 11 o'clock. Three sites of hatching are seen in the embryo in (C). Typical hatching configurations are seen in (D) and (F). The middle row of pictures (G–L) shows embryos in which zonae were opened as illustrated in (G). Embryos (H–L) are hatching through the artificial hole without secondary groups of breaker cells. The zonae remain thick in all cases. The lower row shows embryos after zona thinning. Embryos (M–O) are hatching through non-thinned areas. Artificially thinned areas are indicated by arrows. The embryo in (P) is hatching from three areas and the cells at 2 o'clock are emerging through the thinned area. The embryo in (Q) (and the zona after it was isolated by manipulation) represents an anomaly seen after zona thinning. The embryo is trapped in the zona pellucida after hatching started in an area outside the thinned zone. The scale bar in (A) applies also to (B)–(Q). The scale bar in (R) applies only to (R).
Breaching configurations and time to initial breach and complete hatching
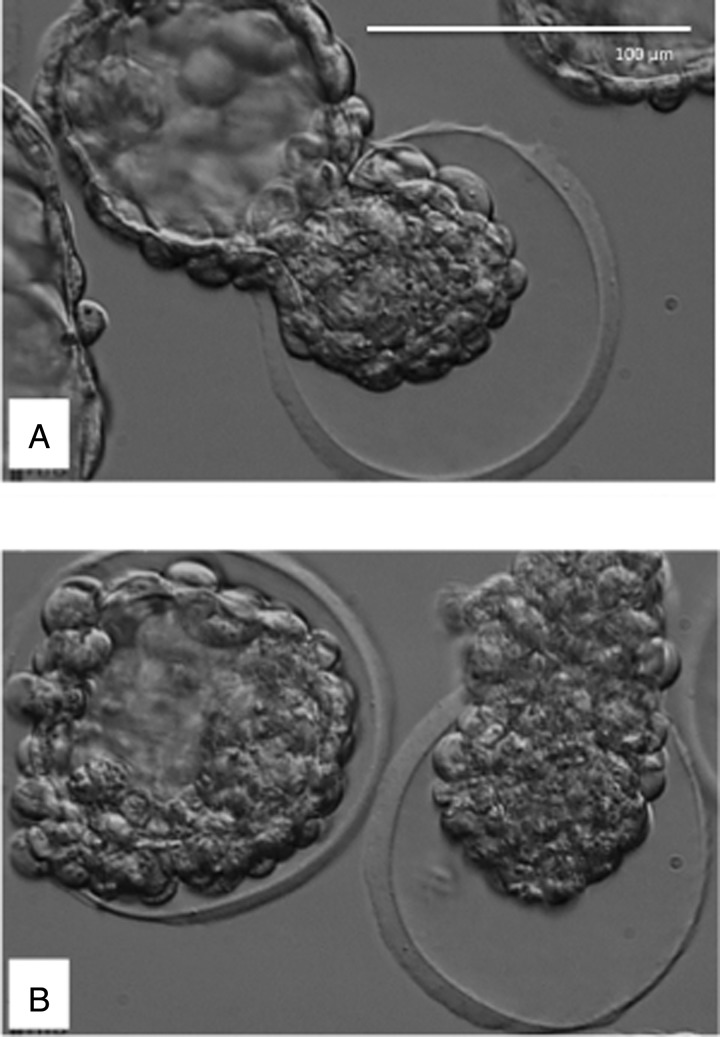
For the purpose of this study, we refer to the first emergence of blastocyst cells through the zona pellucida as ‘breaching’. The frequency of different breaching configurations and the associated hatching outcomes are presented in Table II. Multiple breaching sites were observed in both zona-intact and zona-thinned embryos but not in zona-opened embryos (Fig. 3). Secondary breach sites often involved only a few cells and these either were withdrawn, detached or persisted, the latter leading to entrapment of the embryo within the zona pellucida. The dominant breach site expanded while the other sites contracted.
Table II.
Breaching configurations and outcome of hatching in zona-intact, laser zona-opened and laser zona-thinned mouse embryos, as assessed by TLM.
| Parameter | Group | Single breach N (%) |
Multiple breach N (%) |
|---|---|---|---|
| Partially hatched | Control zona-intact (ZI) (N = 37) | 24 (64.9) | 13 (35.1) |
| Laser zona-opened (ZO) (N = 3) | 3 (100.0) | 0 (0.0) | |
| Laser zona-thinned (ZT) (N = 34) | 17 (50.0) | 17 (50.0) | |
| ZI: single versus multiplea | P = 0.0105 | ||
| ZO: single versus multiple | P = 0.0143 | ||
| ZT: single versus multiple | NS | ||
| Fully hatched | Control zona-intact (ZI) (N = 19) | 14 (73.7) | 5 (26.3) |
| Laser zona-opened (ZO) (N = 51) | 51 (100.0) | 0 (0.0) | |
| Laser zona-thinned (ZT) (N = 22) | 9 (40.9) | 13 (59.1) | |
| ZI: single versus multiple | P = 0.0035 | ||
| ZO: single versus multiple | P < 0.0001 | ||
| ZT: single versus multiple | NS | ||
aT-Test to test pairwise group comparisons.
Figure 3.
Typical response of mouse embryos after zona thinning at the 8-cell stage. Images obtained using an inverted Olympus IX70 equipped with an Octax Adaptive Electronic Condenser (MTG, Germany). (A) The blastocyst hatching opposite the artificially thinned area. (B) The zona pellucida after removal of the blastocyst using suction; multiple openings and thinned area at 3 o’ clock are visible. (C) Typical shape of the opening in the zona after completion of hatching by a zona-thinned embryo that failed to hatch through the thinned area. (D) The thinned area from below.
Time (post hCG) to first breach and to complete hatching in the three groups is presented in Table III. The mean (±SD) number of hours post hCG for completion of hatching in zona-opened versus zona-thinned embryos was 121.3 ± 12.4 and 135.1 ± 14.1 h, respectively (P = 0.0003). The mean number of hours to complete hatching in zona-intact control embryos was 126.8 ± 12.5 h; this was not significantly different from zona-opened or zona-thinned embryos (P > 0.05) (Table III).
Table III.
Time to first breach and to complete hatching in zona-intact, laser zona-opened and laser zona-thinned mouse embryos, as assessed by TLM.
| Group | Control zona-intact (ZI) | Laser zona-opened (ZO) | Laser zona-thinned (ZT) |
|---|---|---|---|
| Time to initial breach Mean hours post hCG (±SD) [Min; Max] |
99.9 (7.2) [88.1; 116.9] |
92.6 (5.5) [81.2; 107.0] |
99.6 (6.4) [86.8; 114.5] |
| Overall group comparisona | |||
| Pairwise comparisonsb | P < 0.0001 | ||
| Control ZI versus ZO | P < 0.0001 | ||
| Control ZI versus ZT | NS | ||
| ZO versus ZT | P < 0.0001 | ||
| Time to complete hatching Mean hours post hCG (±SD) [Min; Max] |
126.8 (12.5) [110.4; 157.5] |
121.3 (12.4) [98.7; 145.0] |
135.1 (14.1) [112.0; 159.8] |
| Overall group comparisona | |||
| Pairwise comparisonsb | P = 0.0003 | ||
| ZI versus ZO | NS | ||
| ZI versus ZT | NS | ||
| ZO versus ZT | P = 0.0003 | ||
aANOVA to test for any group differences.
bT-Test to test pairwise group comparisons.
Hatching configuration with respect to the position of the inner cell mass
TLM allowed examination of the hatching configuration with respect to the position of the ICM. The frequencies with which different hatching configurations occurred are presented in Table IV. The first breach in zona-intact control embryos occurred either at the polar trophectoderm (PTE; 14.5%), i.e. TE cells overlaying the ICM, the proximal mural trophectoderm (PMTE; 43.6%), i.e. midway between polar TE and distal mural TE on the opposite side of the ICM, or at the distal mural trophectoderm (DMTE; 41.8%) and these configurations were similar in zona-thinned embryos (16.1, 48.2 and 35.7%, respectively). In zona-opened embryos, the three hatching configurations occurred roughly with the same frequency. Differences in hatching configuration between the groups did not reach statistical significance (P = 0.1612).
Table IV.
Hatching configuration with respect to ICM position in three groups of embryos.
| Group | PTE N (%) |
PMTE N (%) |
DMTE N (%) |
|---|---|---|---|
| Control zona-intact (ZI)a | 8 (14.6) | 24 (43.6) | 23 (41.8) |
| Laser zona-opened (ZO)b | 17 (31.5) | 18 (33.3) | 19 (35.2) |
| Laser zona- thinned (ZT) | 9 (16.1) | 27 (48.2) | 20 (35.7) |
Hatching with polar trophectoderm (PTE) cells breaching first refers to the inner cell mass (ICM) breaching through the zona pellucida first. Hatching with proximal mural trophectoderm (PMTE) cells refers to the configuration where part of the trophectoderm has moved through the zona pellucida before the ICM escapes and before the distal mural trophectoderm (DMTE) moves out. Mural trophectoderm refers to the configuration where the trophectoderm furthest (opposite) from the ICM moves out of the zona pellucida first, followed by the PMTE and the ICM is the last part of the blastocyst to escape.
Overall group comparisons (ANOVA): NS.
Pairwise comparisons (T-test)
ZI versus ZO: NS.
ZI versus ZT: NS.
ZO versus ZT: NS.
aConfiguration was not assessed in one embryo.
bConfiguration was not assessed in two embryos.
Time to first breach and to complete hatching was determined in all three groups for each hatching configuration (Tables V and VI). Time to first breach was longest when hatching started at PTE and in the zona-thinned group (103.0 ± 7.6 h) (Table V). Time to complete hatching was longest when hatching started at PMTE and in the zona-thinned group (137.5 ± 10.6 h).
Table V.
Hatching configuration and time to first breach in zona-intact, laser zona-opened and laser zona-thinned mouse embryos, as assessed by TLM.
| Time to first breach Mean hours post hCG (±SD) (Min; Max) |
Group |
||
|---|---|---|---|
| Control zona-intact (ZI) | Laser zona-opened (ZO) | Laser zona-thinned (ZT) | |
| Breach site | |||
| PTE | 102.3 (3.3) (98.3; 106.3) |
93.2 (6.9) (85.7; 107.0) |
103.0 (7.6) (95.6; 114.5) |
| Overall group comparisona | |||
| Pairwise comparisonsb | P = 0.0049 | ||
| ZI versus ZO | P = 0.0030 | ||
| ZI versus ZT | NS | ||
| ZO versus ZT | P = 0.0145 | ||
| PMTE | 99.6 (9.1) (88.1; 116.9) |
92.1 (3.8) (86.5; 101.2) |
99.7 (6.2) (89.7; 109.8) |
| Overall group comparisona | |||
| Pairwise comparisonsb | P = 0.0056 | ||
| ZI versus ZO | P = 0.0022 | ||
| ZI versus ZT | NS | ||
| ZO versus ZT | P = 0.0176 | ||
| DMTE | 99.6 (5.2) (88.9; 110.3) |
92.4 (4.7) (81.2; 97.8) |
98.3 (6.1) (86.8; 107.5) |
| Overall group comparisona | |||
| Pairwise comparisonsb | P = 0.0003 | ||
| ZI versus ZO | P = 0.0001 | ||
| ZI versus ZT | NS | ||
| ZO versus ZT | P = 0.0020 | ||
aANOVA to test for group differences.
bT-Test for pairwise group comparisons.
Table VI.
Hatching configuration and time to complete hatching in zona-intact, laser zona-opened and laser zona-thinned mouse embryos, as assessed by TLM.
| Time to complete hatching Mean hours post hCG (±SD) (Min; Max) |
Group |
||
|---|---|---|---|
| Control zona-intact (ZI) | Laser zona-opened (ZO) | Laser zona-thinned (ZT) | |
| ICM position | |||
| PTE | 130.6 (12.6) (121.5; 152.6) |
116.4 (14.2) (99.3; 145.0) |
131.0 (16.5) (117.2; 150.5) |
| Overall group comparisona | |||
| Pairwise comparisonsb | NS | ||
| ZI versus ZO | NS | ||
| ZI versus ZT | NS | ||
| ZO versus ZT | NS | ||
| PMTE | 130.9 (15.8) (116.4 ; 157.5) |
125.2 (10.0) (107.1 ; 144.8) |
137.5 (10.6) (120.2 ; 155.1) |
| Overall group comparisona | |||
| Pairwise comparisonsb | P = 0.0330 | ||
| ZI versus ZO | NS | ||
| ZI versus ZT | NS | ||
| ZO versus ZT | P = 0.0083 | ||
| DMTE | 122.5 (10.4) (110.4; 136.6) |
122.1 (12.0) (98.7; 141.8) |
134.7 (18.0) (112.0; 159.8) |
| Overall group comparisona | |||
| Pairwise comparisonsb | NS | ||
| ZI versus ZO | NS | ||
| ZI versus ZT | NS | ||
| ZO versus ZT | NS | ||
aANOVA to test for group differences.
bT-Test for pairwise group comparisons.
Discussion
The experiments presented here demonstrate that laser-assisted zona thinning in mouse embryos at the 8-cell stage neither assists nor facilitates hatching in vitro. Moreover, based on the significant delay in completion of hatching and frequent appearance of multiple hatching sites which may lead to incomplete hatching, it may be concluded that laser zona thinning can be disruptive to the hatching process.
In contrast, opening the zona pellucida using the same laser facilitates and promotes complete hatching, as has been shown to be the case after zona drilling (Malter and Cohen, 1989). These findings suggest that zona thinning should not be considered a method for assisted hatching.
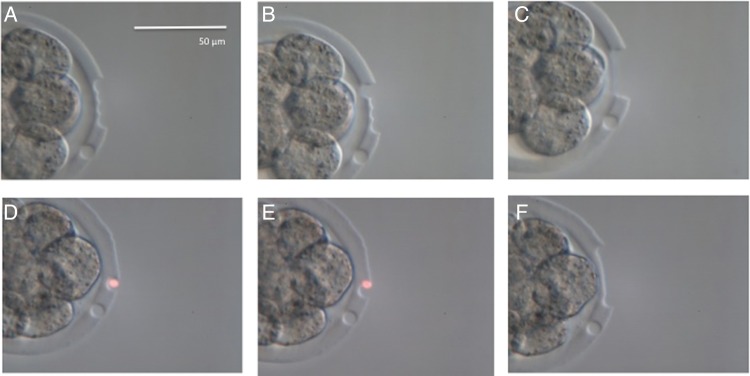
In the current experimental model, the thinned area was limited because the amount of laser energy was kept constant across all groups. When clinically applied, however, the zona is often thinned over a large area spanning one quarter to one half of the zona circumference (Mantoudis et al., 2001; Petersen et al., 2006; Hiraoka et al., 2009). We observed in pilot experiments that the same disruptive alterations in hatching occurred when a method similar to the latter was used in the mouse (Fig. 4) and that embryo development was compromised as a result of the high amount of laser energy used. Notwithstanding the limitations of all animal models, these observations have implications for clinical practice. Most important, the application of zona thinning to human embryos for the purpose of assisted hatching should be re-considered since thinning appears to have no such effect. Second, based on the observed differences in the effect produced by the methods of zona opening and zona thinning, systematic reviews of assisted hatching trials (Das et al., 2009; Martins et al., 2011; Carney et al., 2012) should not combine the results of the two in their evaluations (Cohen and Alikani, 2013).
Figure 4.
Hatching of mouse embryos in which one quarter (A) or half of the zona circumference (B) was thinned, mimicking the method used in the human. Hatching occurred adjacent to the thinned area.
The results of this study are not in agreement with an earlier study on zona thinning using acidified Tyrode's solution in mouse morulae (Khalifa et al., 1992). Those investigators reported a hatching rate of 74% after applying zona thinning, using a two-step approach, resulting in a thinned area shaped like a cross. In the same study, zona drilling (zona opening) resulted in only 43% of the embryos hatching, although this is in sharp contrast to results obtained by Cohen and Feldberg (1991) who applied the same method to cleavage stage embryos; in their experiments, 92% of zona-drilled embryos hatched fully. Comparing the technical details of the two studies does not provide a clear explanation for the differences in results. However, over the years we have attempted zona thinning by acidified Tyrode's solution in the mouse and believe that the procedure is hard to control, often causing inadvertent opening of the zona pellucida over a larger area. Since embryos were not individually examined after micromanipulation in the Khalifa et al. (1992) study, it is possible that cruciate zona thinning caused large but undetected openings in the zona pellucida. This would explain the increase in hatching frequency in the zona-thinned, compared with zona-drilled, embryos. When Khalifa et al. (1992) applied the cruciate zona thinning procedure to human embryos, they did not observe any improvements in clinical outcomes. They attributed the discrepancy between the mouse model and the clinical experience to the physical properties of human zona pellucida, specifically its bilayered organization and concluded that zona thinning was not clinically beneficial.
All embryos in this study were examined using TLM. This technology was particularly useful in deciphering the mechanism of in vitro hatching, as it clearly showed that hatching in about one-third of the zona-intact embryos began with breaches at multiple sites by small groups of cells. This was a surprising finding since it has been generally assumed that the so-called zona breakers penetrate the zona pellucida at only one site—the same site where hatching would eventually occur (Sathananthan et al., 2003). Kirkegaard and co-workers recently observed the hatching process following clinical IVF and ICSI (Kirkegaard et al., 2013). Their observations confirmed Sathananthan's earlier model of hatching, which they described as Type 1, but they also observed a second mechanism (Type 2), which entailed the rupture of the zona following natural progressive thinning, resulting in a wide aperture through which the embryo steadily hatched. Kirkegaard et al. (2013) concluded that Type 1 hatching is more common in human embryos than Type 2. They also noted that Type 1 is the mechanism preferentially used by embryos that resulted from ICSI, suggesting that such embryos may hatch through the small hole created by the sperm injection needle. The authors noted that this apparently did not affect implantation. Both types of hatching were observed in the current experiments as well, suggesting similarities between mouse and human embryo hatching mechanisms in vitro.
Multiple breaches were seen in some human embryos hatching after ICSI, but not after insemination (Kirkegaard et al., 2013). Whether multiple breaches are the result of in vitro culture or they represent a ‘natural’ phenomenon is unknown. In the current experiments, multiple breaches were never detected in the zona-opened embryos while most zona-thinned embryos showed multiple breaching, always involving sites outside the thinned area. Because multiple breaches were also seen in zona-intact embryos, and very little is known about the in vivo process, it cannot be concluded that multiple breaching is an abnormality, per se. Nonetheless, the possibility of trapping following multiple breaching, and a delay in completion of hatching as was seen in these experiments, is of concern, whether the zona is intact or thinned.
Zona opening decreases multiple breaching and allows the blastocyst to escape an average of 14 h earlier compared with zona-thinned embryos. This confirms the findings of Depypere et al. (1988) regarding earlier hatching of zona-drilled embryos. On average, the zona-opened embryos hatched 5.5 h before control embryos. It was shown in the human that zona-drilled embryos implant significantly earlier than their zona-intact counterparts (Liu et al., 1993). Whether earlier hatching provides a clinical advantage is still unclear. Nonetheless, even if the significant shortening of the hatching process after zona opening in the mouse is considered an advantageous alteration of the normal process, it is unclear whether the delay in hatching after zona thinning (on average, 8.5 h later than zona-intact controls) should be considered detrimental but it does suggest a disruption of the process.
Partial or incomplete hatching, also referred to as ‘trapping’ in some publications (Edwards and Purdy, 1982; Cohen and Feldberg, 1991), was seen significantly more frequently in zona-intact and zona-thinned embryos than in zona-opened embryos. Entrapment could safely be assumed to be a deterrent to implantation. Whether alleviation of this problem is a reason for increased pregnancy after assisted hatching is unknown. A previous pilot experiment comparing zona thinning and zona opening in spare human blastocysts only confirmed the relative safety of the use of a non-contact laser (Wong et al., 2003) similar to the one used in the current experiments. Those experiments also compared the hatching process of zona-opened and zona-intact embryos; however, very few zona-thinned embryos were used for logistical reasons. It was shown that hatching was enhanced after zona opening confirming earlier findings (Cohen et al., 1990; Cohen and Feldberg, 1991).
The advantages of TLM for in vitro observation of embryos are multiple and the current study again underscores this. Several observations were made during the current study that would have been difficult using standard microscopy. One of these observations is the multiple zona breaching by zona-breaker cells. Another is that blastocysts do not necessarily attempt to hatch from the artificially thinned area of the zona pellucida, and even if they do, the process is often aborted in favor of another breaching site even though the embryo may finally hatch out of the artificially thinned area. These back and forth hatching attempts are likely one reason the zona-thinned embryos take significantly longer to hatch than either zona-intact or zona-opened embryos.
We also noted differences between the groups in terms of contraction and expansion cycles prior to complete hatching. The highest variability in number of cycles and duration was observed in the zona-thinned embryos, with some embryos showing 50 or more contraction/expansion cycles.
Time-lapse also allowed detailed examination of the hatching configuration. The data suggest that different configurations occur equally frequently (i.e. randomly) in all groups, although the PTE position may be somewhat more common when the zona is opened. Recent studies of assisted hatching in frozen-thawed blastocysts suggest that hatching is enhanced when zona pellucida is opened at the site of the ICM (Miyata et al., 2010). We also observed that hatching is completed faster and more frequently when the PTE (hence the ICM) herniates through the zona pellucida first (data not shown). It was occasionally observed that the ICM showed significant growth when it was to escape last out of the zona; this could potentially result in blockage of the opening and demise of the embryo.
To summarize, the experiments presented here provide evidence that laser-assisted zona thinning in the mouse does not assist hatching. They further provide insight into potential alterations or disruptions in the in vitro hatching process following zona thinning, or indeed when the zona is not manipulated. The findings should therefore cast serious doubt over the clinical utility of zona thinning procedures (Van Wely and van der Veen, 2011) and the validity of the assumption that zona thinning is equivalent to zona opening in assisting hatching of embryos grown in vitro.
Authors' roles
Study was conceived by M.A. T.S. and M.A. performed the initial pilot studies; T.S. and J.C. designed the groups and performed the time-lapse pilot studies. T.S. performed the experiments, made the micrographs and analyzed the annotations. J.C. checked the annotations, performed literature searches and wrote the first draft and the revisions of the paper. The final manuscript was written by M.A., H.S. and J.C. H.S. performed the statistical evaluations and cross-checked the data. M.A. cross-checked the literature searches and edited the manuscript. All authors approved the final manuscript. Revisions were completed by H.S., J.C. and M.A.
Funding
T.S., H.S. and J.C. were funded by Tyho-Galileo Research Laboratory, a division of ART Institute of Washington Inc., Livingston, New Jersey, United States. M.A. is employed by The Center For Human Reproduction, North Shore University Hospital, New York, United States. Funding to pay the Open Access publication charges for this article was provided by ART Institute of Washington.
Conflict of interest
M.A. and H.S. declare no conflict of interest. T.S. is a part-time consultant for Life-Global. J.C. is a founder and shareholder of Reprogenetics LLC. The latter two companies had no involvement in these studies.
References
- Antinori S, Panci C, Selman HA, Caffa B, Dani G, Versaci C. Zona thinning with the use of laser: a new approach to assisted hatching in humans. Hum Reprod. 1996;11:590–594. doi: 10.1093/humrep/11.3.590. [DOI] [PubMed] [Google Scholar]
- Carney SK, Das S, Blake D, Farquhar C, Seif MM, Nelson L. Assisted hatching on assisted conception (in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) Cochrane Database Syst Rev. 2012;12:CD001894. doi: 10.1002/14651858.CD001894.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Alikani M. Evidence-based medicine and its application in clinical preimplantation embryology. Reprod Biomed Online. 2013;27:547–561. doi: 10.1016/j.rbmo.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Cohen J, Feldberg D. Effects of the size and number of zona pellucida openings on hatching and trophoblast outgrowth in the mouse embryo. Mol Reprod Dev. 1991;30:70–78. doi: 10.1002/mrd.1080300110. [DOI] [PubMed] [Google Scholar]
- Cohen J, Elsner C, Kort H, Malter H, Massey J, Mayer MP, Wiemer K. Impairment of hatching process following IVF in the human and improvement of implantation by assisting hatching using micromanipulation. Hum Reprod. 1990;5:7–13. doi: 10.1093/oxfordjournals.humrep.a137044. [DOI] [PubMed] [Google Scholar]
- Cohen J, Alikani M, Trowbridge J, Rosenwaks Z. Implantation enhancement by selective assisted hatching using zona drilling of human embryos with poor prognosis. Hum Reprod. 1992;7:685–691. doi: 10.1093/oxfordjournals.humrep.a137720. [DOI] [PubMed] [Google Scholar]
- Das S, Blake D, Farquhar C, Seif MM. Assisted hatching on assisted conception (IVF and ICSI) Cochrane Database Syst Rev. 2009;2:CD001894. doi: 10.1002/14651858.CD001894.pub4. [DOI] [PubMed] [Google Scholar]
- Depypere HT, McLaughlin KJ, Seamark RF, Warnes GM, Matthews CD. Comparison of zona cutting and zona drilling as techniques for assisted fertilization in the mouse. J Reprod Fertil. 1988;84:205–211. doi: 10.1530/jrf.0.0840205. [DOI] [PubMed] [Google Scholar]
- Edwards RG, Purdy JM. Discussion on the growth of human embryos; Proceedings of the First Bourn Hall Meeting; Academic press; 1982. pp. 214–222. Human conception in vitro. [Google Scholar]
- Edwards RG, Mettler L, Walters DE. Identical twins and in vitro fertilization. J In Vitro Fert Embryo Transf. 1986;3:114–117. doi: 10.1007/BF01139357. [DOI] [PubMed] [Google Scholar]
- Hiraoka K, Hiraoka K, Horiuchi T, Kusuda T, Okano S, Kinutani M, Kinutani K. Impact of the size of zona pellucida thinning area on vitrified-warmed cleavage-stage embryo transfers: a prospective randomized study. J Assist Reprod Genet. 2009;26:515–521. doi: 10.1007/s10815-009-9350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalifa EA, Tucker MJ, Hunt P. Cruciate thinning of the zona pellucida for more successful enhancement of blastocyst hatching in the mouse. Hum Reprod. 1992;7:532–536. doi: 10.1093/oxfordjournals.humrep.a137685. [DOI] [PubMed] [Google Scholar]
- Kirkegaard K, Hindkjaer JJ, Ingerslev HJ. Hatching of in vitro fertilized human embryos is influenced by fertilization method. Fertil Steril. 2013;100:1277–1282. doi: 10.1016/j.fertnstert.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Liu HC, Cohen J, Alikani M, Noyes N, Rosenwaks Z. Assisted hatching facilitates earlier implantation. Fertil Steril. 1993;60:871–875. [PubMed] [Google Scholar]
- Malter HE, Cohen J. Blastocyst formation and hatching in vitro following zona drilling of mouse and human embryos. Gamete Res. 1989;24:67–80. doi: 10.1002/mrd.1120240110. [DOI] [PubMed] [Google Scholar]
- Mantoudis E, Podsiadly BT, Gorgy A, Venkat G, Craft IL. A comparison between quarter, partial and total laser assisted hatching in selected infertility patients. Hum Reprod. 2001;16:2182–2186. doi: 10.1093/humrep/16.10.2182. [DOI] [PubMed] [Google Scholar]
- Martins WP, Rocha IA, Ferriani RA, Nastri CO. Assisted hatching of human embryos: a systematic review and meta-analysis of randomized controlled trials. Hum Reprod Update. 2011;17:438–453. doi: 10.1093/humupd/dmr012. [DOI] [PubMed] [Google Scholar]
- McLaren A. Can mouse blastocysts stimulate a uterine response before losing the zona pellucida? J Reprod Fertil. 1969;19:199–201. doi: 10.1530/jrf.0.0190199. [DOI] [PubMed] [Google Scholar]
- Miyata H, Matsubayashi H, Fukutomi N, Matsuba J, Koizumi A, Tomiyama T. Relevance of the site of assisted hatching in thawed human blastocysts: a preliminary report. Fertil Steril. 2010;94:2444–2447. doi: 10.1016/j.fertnstert.2010.01.056. [DOI] [PubMed] [Google Scholar]
- Petersen CG, Mauri AL, Baruffi RL, Oliveira JB, Felipe V, Massaro FC, Franco JG., Jr Laser-assisted hatching of cryopreserved-thawed embryos by thinning one quarter of the zona. Reprod Biomed Online. 2006;13:668–675. doi: 10.1016/s1472-6483(10)60657-8. [DOI] [PubMed] [Google Scholar]
- Sathananthan H, Menezes J, Gunasheela S. Mechanics of human blastocyst hatching in vitro. Reprod Biomed Online. 2003;7:228–234. doi: 10.1016/s1472-6483(10)61757-9. [DOI] [PubMed] [Google Scholar]
- Tucker MJ, Luecke NM, Wiker SR, Wright G. Chemical removal of the outside of the zona pellucida of day 3 human embryos has no impact on implantation rate. J Assist Reprod Genet. 1993;10:187–191. doi: 10.1007/BF01239219. [DOI] [PubMed] [Google Scholar]
- van Wely M, van der Veen F. To assist or not to assist embryo hatching. Hum Reprod Update. 2011;17:436–437. doi: 10.1093/humupd/dmr019. [DOI] [PubMed] [Google Scholar]
- Wong BC, Boyd CA, Lanzendorf SE. Randomized controlled study of human zona pellucida dissection using the zona infrared laser optical system: evaluation of blastomere damage, embryo development, and subsequent hatching. Fertil Steril. 2003;80:1249–1254. doi: 10.1016/s0015-0282(03)02167-8. [DOI] [PubMed] [Google Scholar]