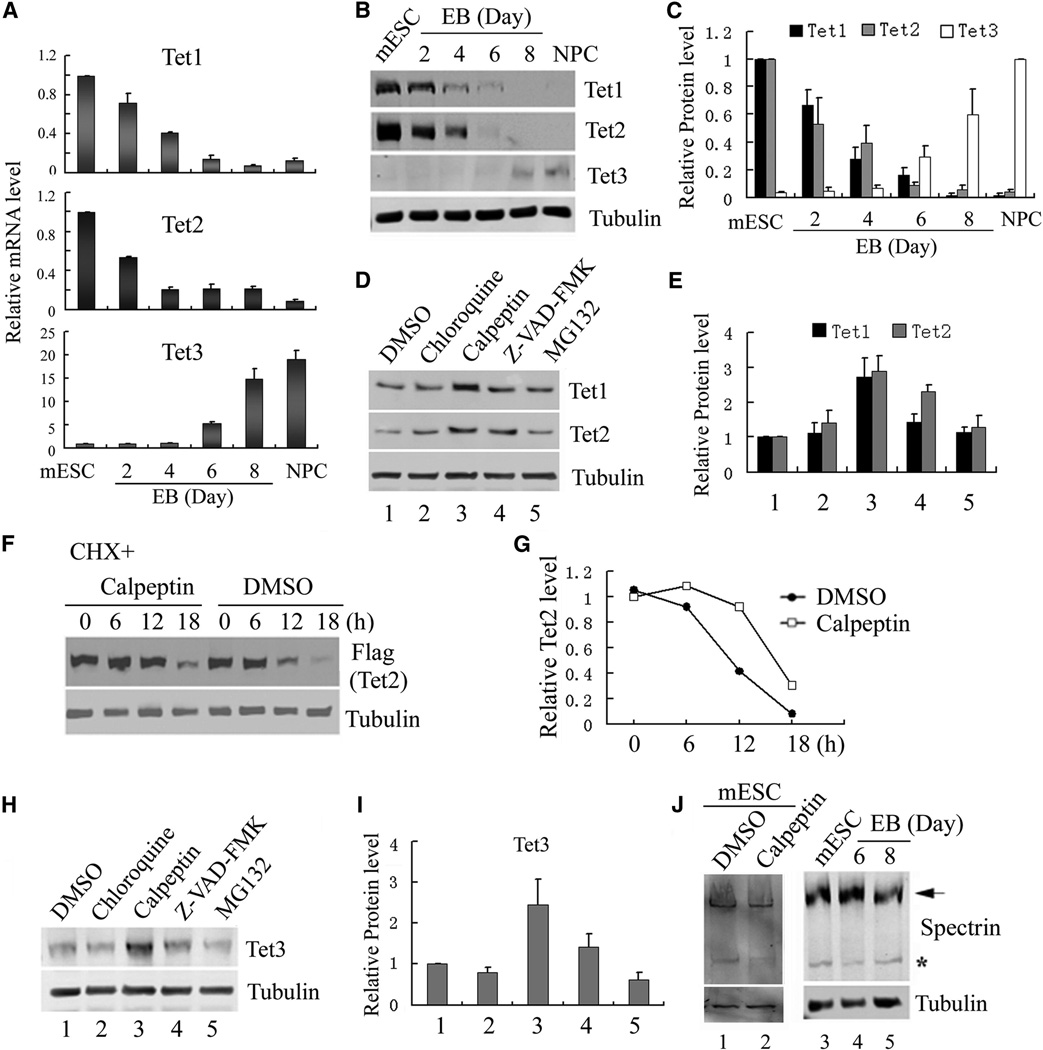
Figure 1. Regulation of TET protein levels by transcription and protein stability.
(A) RT-qPCR analysis of Tet mRNA levels during mESC to NPC differentiation. While Tet1 and Tet2 levels decrease during differentiation, Tet3 level is significantly up-regulated. Data represent the mean of three independent experiments, and Tet levels in mESCs are set as 1.
(B, C) Representative Western blot (B) and quantification of three repeats (C) demonstrate that TET protein levels generally follow mRNA levels during NPC differentiation.
(D, E) Representative Western blot analysis of TET1 and TET2 levels in mESCs treated with chloroquine, calpeptin, Z-VAD-FAM and MG132 for 24h. Quantification of three independent experiments was shown in panel E.
(F, G) Calpeptin increases the half-life of Flag-TET2 protein. Western blot (F) and quantification (G) of the Flag-TET2 levels in the presence or absence of calpeptin upon inhibition of protein translation by cycloheximide.
(H, I) Representative Western blot analysis of TET3 in day 7 embryoid body (EB) treated with chloroquine, calpeptin, Z-VAD-FAM and MG132 for 24h, and the results were quantified in (I).
(J) Calpain activity is detectable in mESCs and during their differentiation. Western blot analysis of mESC lysate with a spectrin antibody identified both full length (arrow) and cleaved spectrin (*), a marker for calpain activity. Spectrin cleavage is detectable during mESC differentiation (lanes 4, 5), and was prevented by calpeptin treatment (compare lane 1 and 2).