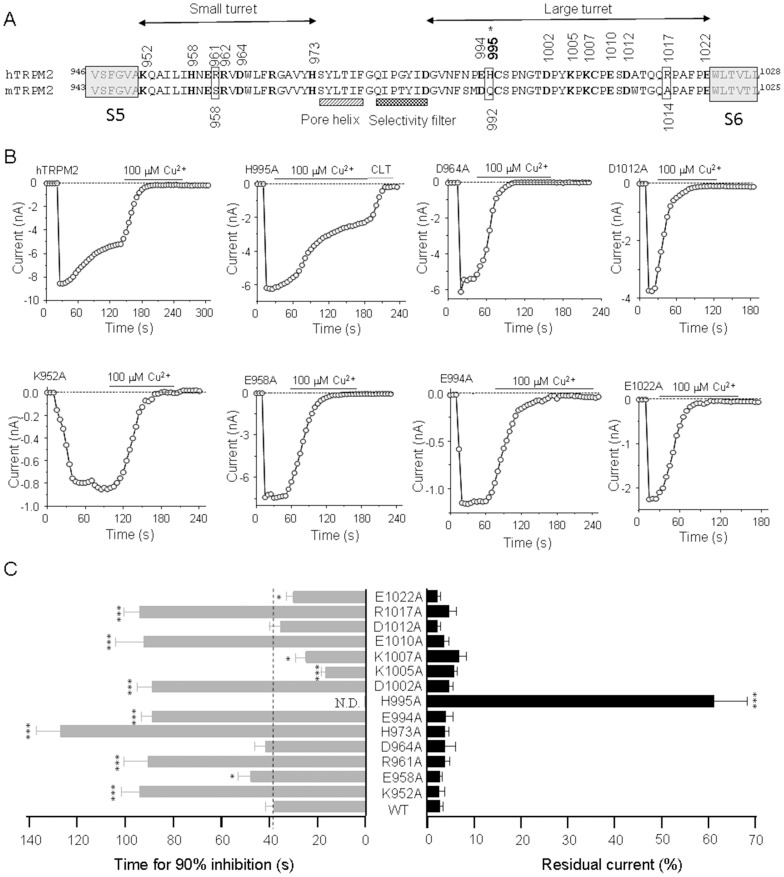
Figure 4. Alanine substitution of Cu2+-binding candidate residues in the outer pore of hTRPM2 channel.
(A) The amino acid sequences of the pore region between the S5 and S6 of the hTRPM2 channel; the residues examined in this study are numbered and highlighted in bold. Residues in the extracellular ends of S5 and S6 are indicated in the left and right shading boxes, respectively. Alanine substitutions leading to loss of function are indicated by asterisks. (B) Representative recordings of the ADPR-induced currents in HEK cells expressing WT or the indicated mutant channel before and after exposure to 100 µM Cu2+ (denoted by the black bars). The currents for the H995A mutant channels show incomplete inhibition by Cu2+ and complete inhibition by subsequent application of 20 µM CLT (denoted by the grey bars). The dotted lines indicate zero currents. (C) Summary of the time for 90% inhibition (left) and the residual currents upon exposure to Cu2+ (right). The dotted lines indicate the time or residual currents for the WT channel. The number of cells examined in each case is 3–22. The mutant channels showing significant difference from the WT channels are indicated in parentheses, *, p<0.05, **, p<0.01, ***, p<0.005.