Abstract
Roseobacticides regulate the symbiotic relationship between a marine bacterium (Phaeobacter inhibens) and a marine microalga (Emiliania huxleyi). This relationship can be mutualistic, when the algal host provides food for the bacteria and the bacteria produce growth hormones and antibiotics for the algae, or parasitic, when the algae senesce and release p-coumaric acid. The released p-coumaric acid causes the bacteria to synthesize roseobacticides, which are nM−μM toxins for the algae. We examined the biosynthesis of roseobacticides and report that all roseobacticide precursors play critical roles during the mutualist phase of the symbiosis. Roseobacticides are biosynthesized from the algal growth promoter, the major food molecule provided by the algal cells, and the algal senescence signal that initiates the mutualist-to-parasite switch. Thus, molecules that are beneficial during mutualism are diverted to the synthesis of toxins during parasitism. A plausible mechanism for assembling roseobacticides from these molecules is proposed.
Naturally occurring small molecules have motivated important chemical studies on structure, synthesis, and biosynthesis, provided therapeutic agents for medicine, and supplied reagents for biology.1,2 While relatively few studies focused on their natural roles, those that did have also revealed interesting chemical and ecological insights. As part of a larger project on the natural roles of bacterially produced small molecules, especially those involved in regulating symbiotic interactions, we uncovered the roseobacticides, which are produced by the marine bacteria Phaeobacter inhibens DSM 17395 and P. inhibens 2.10.3,4 The producers belong to the diverse bacterial roseobacter clade and help regulate the intermittent symbiosis with Emiliania huxleyi,3,5−7 a microscopic single-celled alga that forms massive blooms, synthesizes elaborate calcium carbonate shields, and removes significant quantities of carbon dioxide from the environment through photosynthesis.8,9 This report identifies the biosynthetic precursors for the roseobacticides, which turn out to be hybrid molecules composed of fragments contributed by both the algae and the bacteria.
Recent studies by several research groups have shown that roseobacters engage in a dynamic and opportunistic symbiosis with microalgal cells.3,10,11 The algae provide food in the form of dimethylsulfoniopropionate (Figure 1, DMSP, 1), which P. inhibens can use as a source of sulfur and carbon. In return, the bacteria produce tropodithietic acid (TDA, 2),12−14 a broad-spectrum antibiotic that protects against pathogenic bacteria, and phenylacetic acid (3), an algal growth hormone. In certain interactions, a number of vitamins, notably vitamin B12, have also been implicated.15,16 This mutually beneficial relationship changes in the presence p-coumaric acid (pCA, 4), a potential senescence signal produced by the algal host.3 In response to pCA, the bacteria activate an otherwise silent biosynthetic pathway that generates the algaecidal roseobacticides (5–7). While the small molecules involved in this symbiosis have been identified, the biosynthetic pathways that produce them remain unknown.
Figure 1.
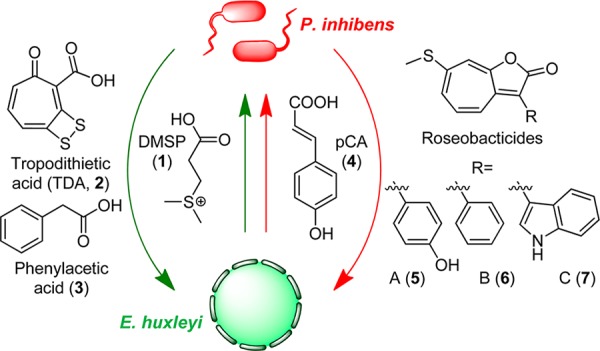
Model for metabolite exchange in the algal-bacterial symbiosis between E. huxleyi and P. inhibens. The symbiotic interaction comprises two phases, a mutualistic phase (green arrows) and a parasitic phase (red arrows). In the mutualistic phase, E. huxleyi provides the C- and S-source 1. The bacteria in return produce 2, an antibiotic that protects the host from bacterial pathogens, and 3, an algal growth promoter. In the parasitic phase, P. inhibens generates the algaecidal roseobacticides (5–7) in response to 4, a likely senescence signal produced by E. huxleyi.
The recent discovery of 11 roseobacticide analogs suggested that they may be generated from tropone, an aromatic amino acid (Phe, Tyr, or Trp), and MeSH (in the case of analogs A-C).4 To examine these hypotheses, we initiated a series of isotope feeding experiments coupled with multidimensional NMR and high-resolution MS (HR-MS) to uncover the precursors of roseobacticides. Elegant studies by Schulz et al. recently showed that P. inhibens can convert Phe into tropone or tropone hydrate (i.e., 5-cycloheptene-1,3-dione).17 As shown in Scheme 1, these compounds are generated with different (effective) planes of symmetry. Using 1,2-13C2-phenylacetic acid (8) as a substrate, the pathway would provide 2-13C-tropone (9), which is asymmetrically labeled and would be expected to give two isotopomers of roseobacticide B. In contrast, 1,2-13C2-phenylacetic acid yields 5-cycloheptene-1,3-dione labeled at the unique C2 position (10) and would thus result in roseobacticide B with a single isotopomer.
Scheme 1. Expected Patterns of Isotope Incorporation into Roseobacticide B from 1,2-13C- and 11.
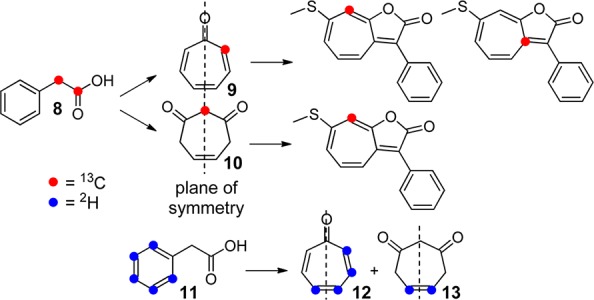
To distinguish between these options, P. inhibens DSM 17395 was cultured in YTSS media supplemented with 0.4 mM 1,2-13C2-phenylacetic acid. Production of roseobacticides was induced by addition of sinapic acid and roseobacticide B subsequently isolated as previously described4 and assessed by HR-MS and 2D NMR. The former gave [M + H]+expt 270.0673 consistent with incorporation of a single 13C ([M + H]+calc 270.0670, Table S1). 13C NMR, gHSQC, and gHMBC spectra demonstrated labeling at a single position, the C8 of roseobacticide B, entirely consistent with 5-cycloheptene-1,3-dione, but not tropone, serving as a precursor for roseobacticide B (Figures 2 and S1). These results were further corroborated using ring-2H5-phenylacetic acid (11). Thiel et al. have reported that 11 is converted to 12 and 13, again demonstrating an asymmetric distribution of 2H in tropone versus symmetric labeling in the 5-cycloheptene-1,3-dione, in which solvent exchange leads to loss of deuterons α to the carbonyl groups.17 Isolation of roseobacticide B from production cultures grown in the presence of 11 yielded an isotopomer containing two deuterons ([M + H]+calc 271.0762, [M + H]+expt 271.0745). These results indicate that 3, a growth stimulant produced during the beneficial phase of the symbiosis, is employed as a precursor for toxin production in the parasitic phase.
Figure 2.
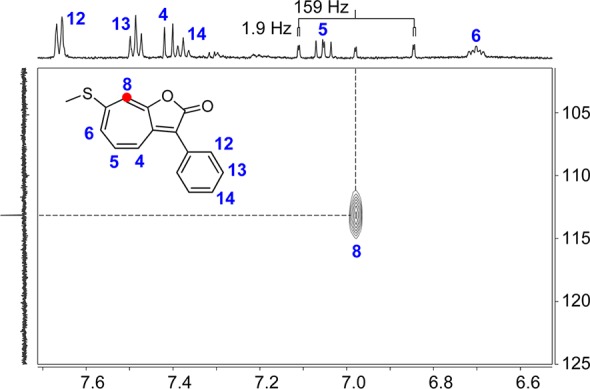
Incorporation of an algal growth promoter into roseobacticide B. Shown is the gHSQC spectrum of 5 isolated from P. inhibens cultures containing compound 8. A single correlation is observed corresponding to 13C insertion at the C8 position of roseobacticide B. As expected, a C–H coupling constant of 159 Hz is observed at C8. Labeled roseobacticide B is shown along with the assigned shifts in the 1H trace.
Interestingly, neither 2H nor 13C from phenylacetic acid was incorporated into the aromatic substituent of roseobacticide B. A similar result was obtained with 2H5-indoleacetic acid indicating that the side chain is not derived from phenylacetic acid or indoleacetic acid, in the cases of roseobacticides B and C, respectively. To illuminate the source of the aromatic side chain, deuterated and 13C-containing amino acids were employed. P. inhibens DSM 17395 cultures were grown in the presence of ring-2H5-Trp and roseobacticide C isolated and characterized. Comparison of the 1H NMR spectra in Figure 3A,B clearly shows a disappearance of the indole ring protons consistent with incorporation of 2H5-indole into roseobacticide C. These results are further confirmed with HR-MS data, which yield [M + H]+expt 313.1063 ([M + H]+calc 313.1059). Analogous experiments were carried out with ring-2H4-Tyr, 2-13C-Tyr, and ring-2H5-Phe. The Tyr analogs revealed incorporation of the 2H4-phenol into roseobacticide A as well as placement of the α-carbon of Tyr as the carbonyl-C2 of roseobacticide A (Figure 3C, Scheme 2A, Figure S2). Experiments with ring-2H5-Phe gave three distinct labeling patterns, containing either 2, 5, or 7 deuterons (Figure 3D and Scheme 2B). NMR and HR-MS analyses revealed that these consisted of either two deuterons in the tropone moiety, five in the phenyl group, or the combination of these two to yield 2H7-roseobacticide B (Figure S3). These results establish the role of Phe as the source of both the tropone moiety and the aromatic side chain in roseobacticide B as well as those of Tyr and Trp in generating the other roseobacticide analogs.
Figure 3.
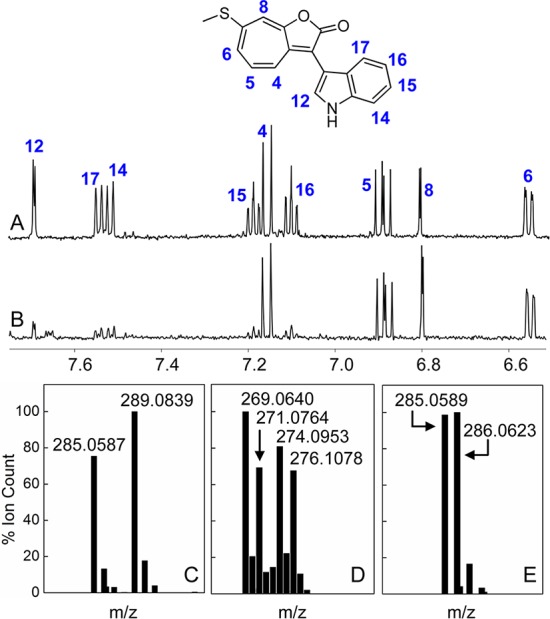
Aromatic amino acids and signal pCA serve as roseobacticide precursors. (A) Assigned 1H NMR spectrum of roseobacticide C. (B) 1H spectrum of roseobacticide C isolated from cultures containing ring-2H5-Trp, showing incorporation of 2H5-indole. (C) HR-MS spectrum of roseobacticide A isolated from cultures containing ring-2H4-Tyr. Both protonated and 2H4-phenol-bearing isotopomers are observed. (D) HR-MS spectrum of roseobacticide B isolated from cultures containing ring-2H5-Phe. Three deuterated isotopomers and the all-protonated form are observed. (E) HR-MS spectrum of roseobacticide A isolated from cultures containing 2-13C-pCA showing incorporation of signal pCA into roseobacticide A; see Scheme 2 and Table S1.
Scheme 2. Observed Patterns of Isotope Incorporation for a Number of Labeled Precursor Molecules.
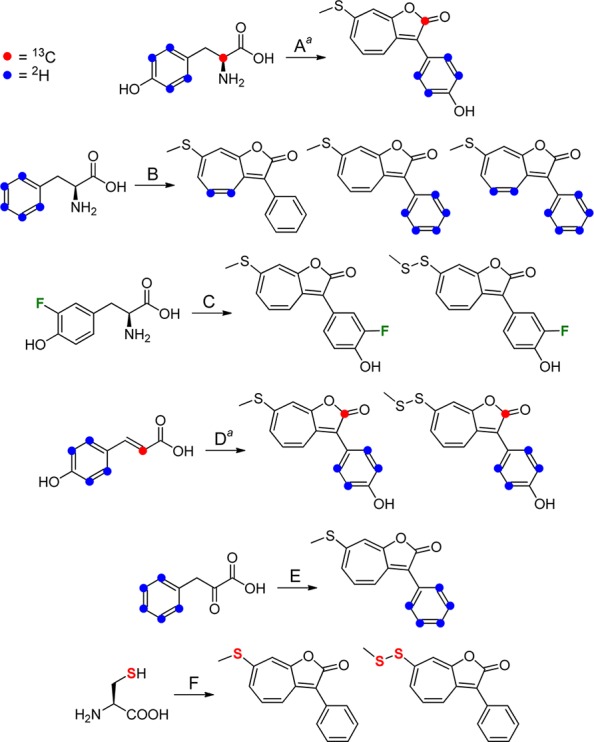
Experiments with 2H4- and 13C-labeled precursors were carried out separately in reactions A and D but are combined above for brevity.
Given that amino acids serve as roseobacticide precursors, we wondered whether this knowledge could be used to generate new analogs and whether pCA, which could be converted to Tyr via the enzyme tyrosine ammonia lyase,18 might be incorporated into roseobacticide A. This incorporation would represent an unusual scenario in which the algal signal that activates the roseobacticide biosynthetic pathway also serves as a substrate for its synthesis. We tested these hypotheses by performing production cultures with ring-2H4-pCA, 2-13C-pCA, and 3-fluorotyrosine. The latter was commercially available, and cultures containing 3-F-Tyr indeed gave rise to fluorinated roseobacticides A and D, as demonstrated by 1H, 19F, and gCOSY NMR spectra and by HR-MS studies (Scheme 2C, Figure S4, Tables S1 and S2). These results highlight the flexibility of the enzymes in the roseobacticide biosynthetic pathway that can accommodate a fluorine substituent on the Tyr substrate. To test whether signal pCA might be incorporated, ring-2H4-pCA and 2-13C-pCA were synthesized by a Knoevenagal condensation of the appropriately labeled malonic acid and 4-hydroxybenzaldehyde isotopomers (see SI).19 Surprisingly, NMR and HR-MS analysis of roseobacticide A and D clearly showed incorporation of the 2H4-phenol group of ring-2H4-pCA into the side chain as well as insertion of the 2-13C in pCA as the 2-13C carbonyl in roseobacticide A (Figure 3E, Scheme 2D, Figure S5). In the natural system, local concentrations of pCA are not known as the bacterial cells adhere to an algal cell. In our algal-free system, pCA signaling and pCA-incorporation into roseobacticides both occur at the same concentration, >0.4 mM.3,4 Collectively, the data above indicate that pCA serves both as a trigger and a substrate for roseobacticides, which contain a phenol group at position C3, and that the bacteria incorporate the algal signal pCA into the algal toxin.
A number of plausible mechanisms may be proposed for the incorporation of aromatic amino acids into roseobacticides. Incorporation of the α-carbon of Tyr as the C2-carbonyl of roseobacticide A suggests that the Tyr carboxyl group is lost during the course of biosynthesis (Scheme 2A). The absence of the amino group necessitates a deaminated analog as the direct precursor. Accordingly, we tested incorporation of several products of the Phe/Tyr degradation pathway. One such product is the deaminated phenylpyruvic acid, generated from Phe by a putative aminotransferase (PGA1_c29420), which can be found in the genome of P. inhibens DSM 17395. Ring-2H5-phenylpyruvic acid was synthesized enzymatically as previously described.20 Inclusion of this compound in production cultures of P. inhibens DSM 17395 clearly demonstrated incorporation of the 2H5-phenyl group into roseobacticide B (Scheme 2E, Table S1). Two other potential substrates, ring-2H5-phenylglycine, obtained commercially, and ring-2H5-phenylglyoxylic acid, synthesized by SeO2-mediated oxidation of the corresponding ring-2H5-phenylacetic acid,21 both failed to incorporate into roseobacticides. The isotope feeding results regarding the side chain of roseobacticides may best be explained by the pathway in Scheme 3. In this pathway, Phe is converted to phenylpyruvate by an aminotransferase. Ior1 (PGA1_c04490), recently identified,16,22 then catalyzes conversion of phenylpyruvate to phenylacetyl-CoA. Thus, phenylacetic acid is not an intermediate in this pathway perhaps explaining the lack of incorporation into the side chain of roseobacticides. BLAST searches against the genome of P. inhibens DSM 17395 also revealed an enzyme complex that has been shown to generate phenylglyoxylyl-CoA from phenylacetyl-CoA (PGA1_c28900), suggesting that either may serve as the direct precursor to the roseobacticides (see below).23
Scheme 3. Proposed Pathway for Production of Phen-ylglyoxylyl-CoA in P. inhibens DSM 17395.
a: PGA1_c29420; b: PGA1_c04490; c: PGA1_c28900.
The experiments above have focused on the source of the seven-membered ring and the side chain in roseobacticides. We next addressed the source of the thiomethyl group in 5–7. P. inhibens has been shown to synthesize a number of small, volatile sulfur-containing compounds, some with highly unusual structures.24,25 In a natural context, DMSP (1, Figure 1) is likely the only sulfur source, which can be converted to Cys.26 To examine a role for Cys in the production of roseobacticides, P. inhibens DSM 17395 cultures were supplemented with 34S-Cys.27 HR-MS data convincingly show incorporation of 34S into roseobacticides B and E revealing that the sulfur atoms in the thiomethyl and methyl disulfide substituents are provided by Cys (Scheme 2F, Figure S6). Thus, these results suggest that DMSP, the food molecule provided by the algal host, is utilized by the bacteria as a substrate to generate the algal toxin.
The results from the isotope feeding data above may be integrated to propose a biosynthetic pathway for roseobacticides (Figure 4). Three amino acids, Tyr, Phe and Cys, are the precursors to roseobacticide A. Phe provides phenylacetic acid, and Cys, derived from the food molecule DMSP, affords the sulfur atoms(s) (Figure 4A). Tyr provides the side chain as well as part of the five-membered ring lactone and may be derived from Phe or from pCA, a conversion catalyzed by the enzyme Tyr ammonia lyase (TAL). BLAST searches in P. inhibens DSM 17395 reveal an enzyme with high homology to the E. coli TAL, putatively annotated as a His ammonia lyase (PGA1_c36340). In roseobacticides B and C, Tyr is replaced with Phe and Trp, respectively. In our current working model (Figure 4B), a 5-cycloheptene-1,3-dione carbanion attacks the α-ketone carbonyl of an aromatic glyoxylyl-CoA (15) to give 16. Lactone formation is driven by the loss of CoA forming 17. DMSP then, most likely via Cys, provides the sulfur for the thiomethyl group by an unknown mechanism, perhaps akin to that recently proposed for sulfur insertion into TDA.27 Additional experiments will be necessary to test this proposed pathway, and studies addressing the genetics and enzymology of roseobacticide biosynthesis are currently underway.
Figure 4.
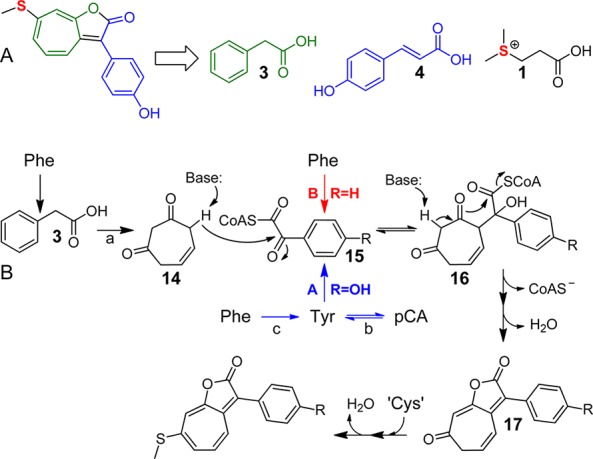
Precursors and proposed pathway for roseobacticide biosynthesis. (A) Roseobacticide A may be synthesized from two algal molecules (1 and 4) and from the algal growth promoter (3) used during mutualism. (B) Phe may be converted to 3 and 5-cycloheptene-1,3-dione (14) via pathway (a), previously described.17 Tyr may be provided by a putative TAL (PGA1_c36340, reaction b) or from Phe (reaction c). See text for a description.
In conclusion, the roseobacticides are assembled from three molecules (1, 3, 4) that play key roles in the symbiosis between members of the roseobacter and a marine alga (Figure 4A). Phenylacetic acid (3) is a bacterially produced algal growth promoter, p-coumaric acid (4) is an algal senescence molecule, and Cys is derived from algal DMSP (1), which nourishes the bacteria. All three are combined by P. inhibens to generate the roseobacticides. Thus, beneficial molecules in the mutualistic phase are converted into toxins in the parasitic phase, a remarkable example of metabolic economy. This economy likely reflects both the nutrient-poor environment in which the symbiosis occurs and the necessity for a switch-like conversion. Finally, our studies illustrate that defining biosynthetic pathways not only describes a set of chemical reactions but can also provide insights into the molecular dialogue governing symbiotic interactions.
Acknowledgments
We thank Prof. Jeroen Dickschat for the kind gift of 34S-Cys, Dr. Istvan Pelczer at the Princeton Chemistry NMR facility for assistance with NMR data acquisition, and grants from the National Institutes of Health (GM098299 to M.R.S., GM086258 to J.C., and GM82137 to R.K.) for support of this work.
Supporting Information Available
Experimental methods and analytical data. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Clardy J.; Walsh C. T. Nature 2004, 432, 829. [DOI] [PubMed] [Google Scholar]
- Meinwald J. J. Nat. Prod. 2011, 74, 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyedsayamdost M. R.; Case R. J.; Kolter R.; Clardy J. Nat. Chem. 2011, 3, 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyedsayamdost M. R.; Carr G.; Kolter R.; Clardy J. J. Am. Chem. Soc. 2011, 133, 18343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng H.; Belas R. Curr. Opin. Biotechnol. 2010, 21, 332. [DOI] [PubMed] [Google Scholar]
- Wagner-Döbler I.; Biebl H. Annu. Rev. Microbiol. 2006, 60, 255. [DOI] [PubMed] [Google Scholar]
- Buchan A.; Gonzalez J. M.; Moran M. A. Appl. Environ. Microbiol. 2005, 71, 5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel D. A.; Franz B. A. Nature 2010, 466, 569. [DOI] [PubMed] [Google Scholar]
- Holligan P. M.; et al. Global Biogeochem. Cycles 1993, 7, 879. [Google Scholar]
- Sule P.; Belas R. J. Bacteriol. 2013, 195, 637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.; Tomasch J.; Jarek M.; Wagner-Döbler I. Front. Microbiol. 2014, 5, 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhn J. B.; Nielsen K. F.; Hjelm M.; Hansen M.; Bresciani J.; Schulz S.; Gram L. Appl. Environ. Microbiol. 2005, 71, 7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer E. M.; Aebisher D.; Greer A.; Bentley R. J. Org. Chem. 2008, 73, 280. [DOI] [PubMed] [Google Scholar]
- Geng H.; Bruhn J. B.; Nielsen K. F.; Gram L.; Belas R. Appl. Environ. Microbiol. 2008, 74, 1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft M. T.; Lawrence A. D.; Raux-Deery E.; Warren M. J.; Smith A. G. Nature 2005, 438, 90. [DOI] [PubMed] [Google Scholar]
- Wagner-Döbler I.; et al. ISME J. 2010, 4, 61.19741735 [Google Scholar]
- Thiel V.; Brinkhoff T.; Dickschat J. S.; Wickel S.; Grunenberg J.; Wagner-Döbler I.; Simon M.; Schulz S. Org. Biomol. Chem. 2010, 8, 234. [DOI] [PubMed] [Google Scholar]
- MacDonald M. J.; D’Cunha G. B. Biochem. Cell Biol. 2007, 85, 273. [DOI] [PubMed] [Google Scholar]
- a Robbins R. J.; Schmidt W. F. J. Labelled Compd. Radiopharm. 2004, 47, 797. [Google Scholar]; b Skowera K.; Kanska M. J. Labelled Compd. Radiopharm. 2008, 51, 321. [Google Scholar]; c Murata S.; Sugiyama K.; Tomioka H. J. Org. Chem. 1993, 58, 1976. [Google Scholar]
- Berger M.; Brock N. L.; Liesegang H.; Dogs M.; Preuth I.; Simon M.; Dickschat J.; Brinkhoff T. Appl. Environ. Microbiol. 2012, 78, 3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S.-K.; Fuchs G. Eur. J. Biochem. 1999, 262, 507. [DOI] [PubMed] [Google Scholar]
- a Brock N. L.; Menke M.; Klapschinski T. A.; Dickschat J. S. Org. Biomol. Chem. 2014, 12, 4318. [DOI] [PubMed] [Google Scholar]; b Dickschat J. S.; Zell C.; Brock N. L. ChemBioChem 2010, 11, 417. [DOI] [PubMed] [Google Scholar]
- Kiene R. P.; Linn L. J.; Gonzalez J.; Moran M. A.; Bruton J. A. Appl. Environ. Microbiol. 1999, 65, 4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock N. L.; Nikolay A.; Dickschat J. S. Chem. Commun. 2014, 50, 5487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


