Figure 1.
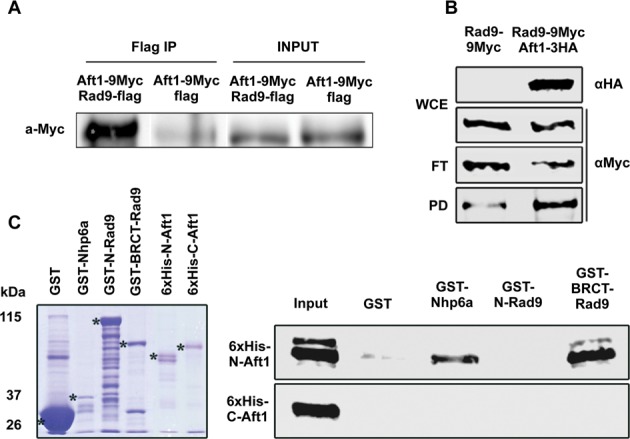
Rad9 associates with Aft1 in vivo with its BRCT domain interacting with N-Aft1 in vitro.(A) In rad9Δ background, Aft1 was tagged with 9Myc epitopes. Rad9 tagged with flag epitope was inserted in the high copy plasmid pDB20 and this construct was inserted to rad9Δ strain in order to get rad9Δ Aft1–9Myc pDB20-Rad9-flag strain. rad9Δ Aft1–9Myc pDB20-flag strain was used as control. In SDS-PAGE and immunoblotting analysis, anti-Flag was used as a bait and probing was done with anti-Myc. Input and IP samples are shown. The co-IP experiment was repeated twice. (B) Protein extracts were prepared from wt cells grown in YPD/BPS-BCS, endogenously expressing Rad9–9Myc or Rad9–9Myc along with Aft1–3HA. Portions (1/40) of each extract (WCE) were analyzed by SDS-PAGE and immunoblotting using anti-HA or anti-Myc to detect Aft1–3HA and Rad9–9Myc, respectively. Remaining extracts were incubated with EZview anti-HA agarose beads and then flow-through (FT) as well as pulled-down (PD) proteins were analyzed by SDS-PAGE and immunoblotting using anti-Myc to detect Rad9–9Myc. (C) Bacterially expressed GST (negative control), GST-Nhp6a (positive control) (32), GST-N-Rad9 or GST-BRCT-Rad9 proteins, bound on glutathione agarose beads, were incubated with bacterially expressed 6xHis-N-Aft1 or 6xHis-C-Aft1 derivatives eluted from Ni-NTA agarose beads. Glutathione beads were then washed and proteins bound on them were analysed by SDS-PAGE and immunoblotting using anti-His antibody. The input lane contains 20% of the total amount of each 6xHis-tagged protein incubated with the beads. Left panel: coomassie blue gel showing the electrophoretic pattern of the GST-tagged (total amounts) as well as the 6xHis-tagged (input amounts) proteins used in the assay.
