Figure 3.
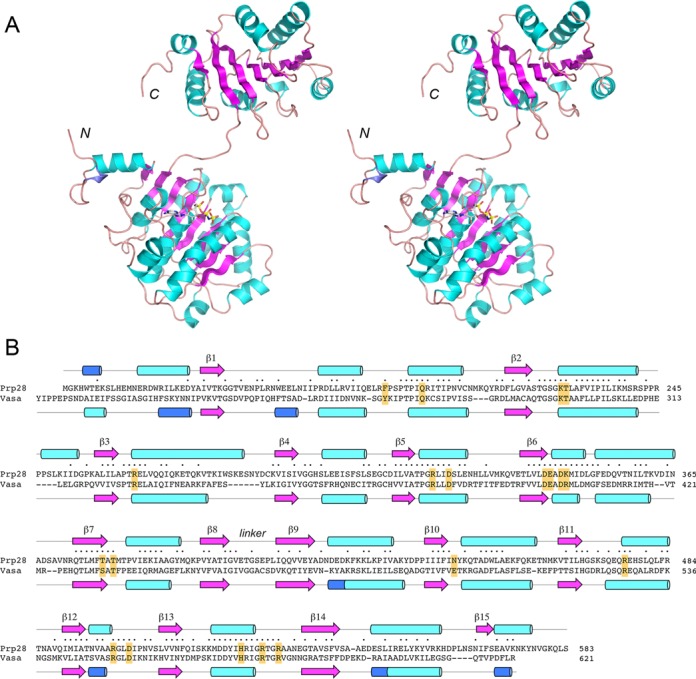
Structure of yeast Prp28-ΔN and homology to Vasa. (A) A stereo view of the tertiary structure of the B protomer of Prp28-ΔN is shown as a cartoon model, with β strands colored magenta, α helices colored cyan and 310 helices colored blue. The N and C termini are indicated. AMPPNP bound to the N domain is depicted as a stick model. (B) The secondary structure elements of yeast Prp28 and Drosophila Vasa (colored as in panel A) are shown above and below their aligned amino acid sequences, with β strands rendered as arrows and helices as cylinders. Gaps in the alignment are indicated by dashes. Positions of amino acid side chain identity/similarity are indicated by • above the sequence. The conserved amino acids in Prp28 that were target for alanine scanning in Prp28 are highlighted in yellow boxes.
