Figure 3.
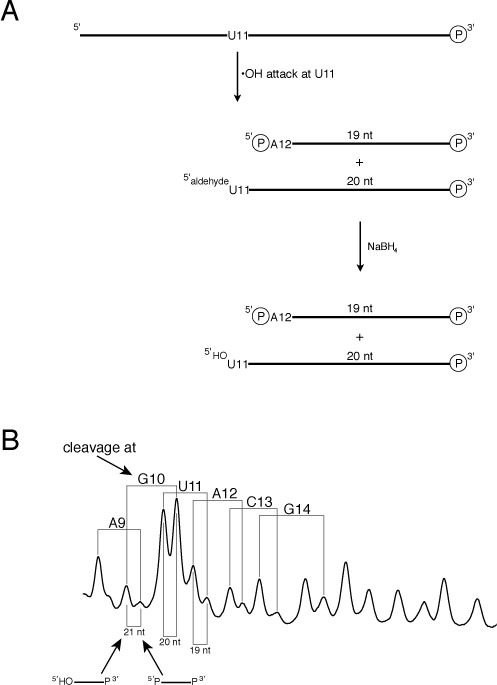
Two hydroxyl radical cleavage products are observed for each nucleotide when the SRL is 3′-radiolabeled. (A) For 5′-hydrogen abstraction the attacked base and sugar are still present in the product (5′-hydroxyl terminus), while for abstraction of other hydrogen atoms the base is released and the sugar is destroyed, yielding a product one nucleotide shorter (5′-phosphate terminus). Note that the lengths of RNA fragments include the extra C that is added during 3′-radiolabeling (see the Materials and Methods section). (B) Cleavage products were treated with sodium borohydride and electrophoresed on a denaturing gel. Assignments of representative product lengths are shown below the scan. (Note that the lengths of RNA fragments include the extra C that is added during 3′-radiolabeling.) The left-most peak in a pair corresponds to an RNA molecule terminated by 5′-hydroxyl; the right-most peak corresponds to an RNA molecule terminated by 5′-phosphate. Assignments of SRL residues at which hydrogen atom abstraction occurred are shown above the scan. For each nucleotide that was attacked, two peaks are assigned. The lower mobility fragment (left) is the product of 5′-hydrogen abstraction and is terminated by 5′-hydroxyl; the higher mobility fragment (right) is the product of abstraction of one of the other ribose hydrogens and is terminated by 5′-phosphate.
