Figure 6.
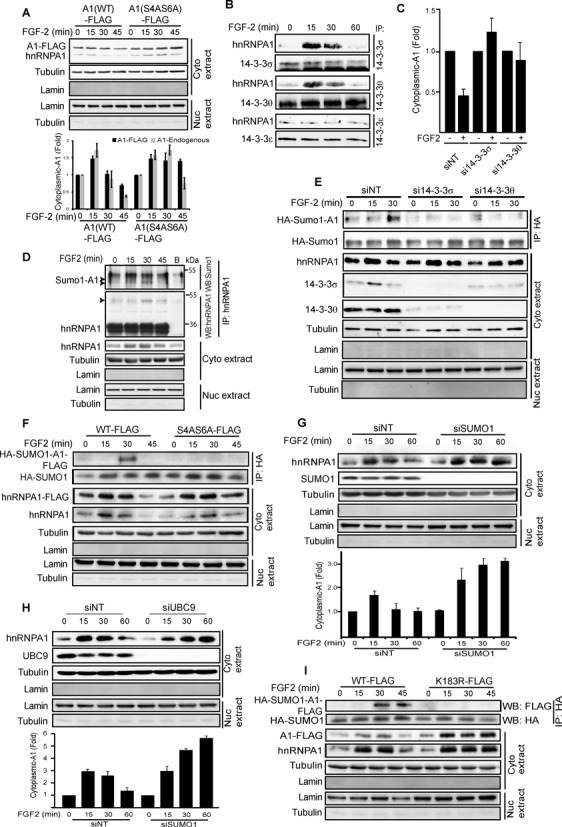
Cytoplasmic hnRNPA1 associates with 14-3-3 subunits and is sumoylated upon stimulation with FGF-2. (A, C, G and H) HEK293 cells were transiently transfected with (A) wild-type (WT) or S4AS6A mutant hnRNPA1-FLAG or (C, G and H) siRNAs against the indicated proteins or a non-targeting control (NT) were treated ± FGF-2 for the indicated time (A and G) or 60 min (C) and the cytoplasmic fraction analysed by SDS-PAGE/western blotting (WB). (A, G and H lower panels and C) Band intensities for hnRNPA1 were quantified and normalized to that for tubulin. Results show average of triplicate experiments ± SEM normalised to the corresponding controls. In Fig6A, lower panel shows quantification for both endogenous and the FLAG-tagged hnRNPA1. (B) HEK293 cells were treated with FGF-2 and the endogenous 14-3-3 protein subunits σ, θ or ϵ immunoprecipitated (IP) from the cytoplasmic fraction. Immunoprecipitates were analysed by SDS-PAGE/WB for endogenous hnRNPA1 or 14-3-3 proteins. (D) Endogenous hnRNPA1 was immunoprecipitated from the cytoplasmic fraction of HEK293 cells treated ± FGF-2. Immunoprecipitates were analysed by SDS-PAGE/WB with either Sumo1 or hnRNPA1 antibodies. The arrows indicate sumoylated-hnRNPA1. (E) HEK293 cells stably expressing HA-Sumo1 were treated with siRNA for 14-3-3σ, θ or NT before stimulation with FGF-2. HA-SUMO1 was immunoprecipitated with anti-HA antibodies from the cytoplasmic fraction and the samples analysed by SDS-PAGE/WB for the indicated proteins. (F and I) HEK293 cells stably expressing HA-SUMO1 were transiently transfected with FLAG-tagged WT or S4AS6A or K183R mutant hnRNPA1 and treated with FGF-2. Cytoplasmic fractions were immunoprecipitated for HA-SUMO1 and analysed as above. All experiments are representative of three independent experiments. See also Supplementary Figure S3.
