Figure 8.
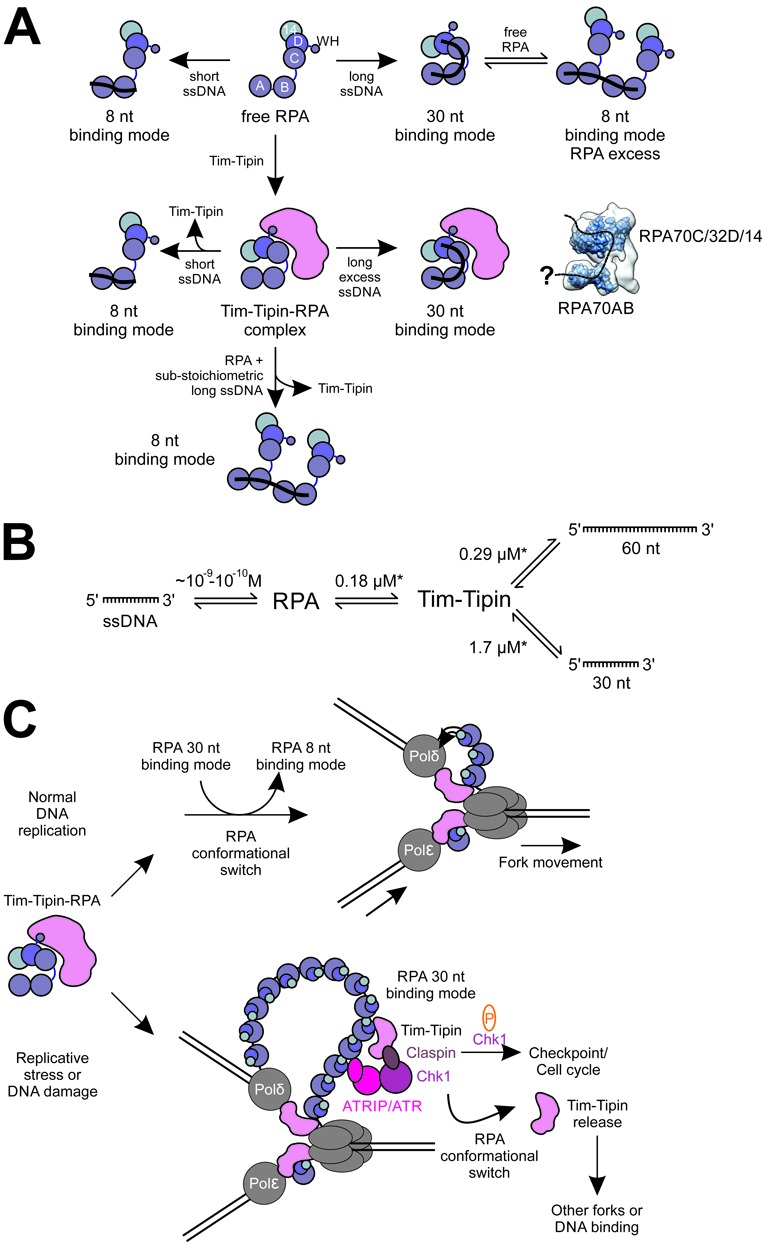
Tim-Tipin-RPA complex formation relies on ssDNA length-dependent RPA conformations. (A) Graphical scheme of the dynamic formation and disruption of the Tim-Tipin-RPA-ssDNA complex in connection with the ssDNA-length dependent conformational change of RPA. RPA shows two binding modes (8 and 30 nt binding mode), which can coexist in a dynamic equilibrium in solution (top). RPA and Tim-Tipin form a complex without and in the presence of long excess ssDNA. In the DNA-free state, the RPA conformation is fixed by Tim-Tipin into a compact mode resembling RPA's conformation in the presence of long ssDNA. The Tim-Tipin-RPA-ssDNA complex dissociates in the presence of short or substoichiometric amounts of long ssDNA, thus upon the conformational change of RPA to the 8 nt binding mode. A possible ssDNA path within our Tim-Tipin-RPA EM reconstruction is indicated as dashed line. (B) Interaction scheme of RPA and ssDNA, RPA and Tim-Tipin and Tim-Tipin and ssDNA showing the KD determined in this study (marked by star). RPA shows a very high (nano- to subnanomolar) affinity to ssDNA in comparison to Tim-Tipin (micromolar) suggesting that Tim-Tipin binding to ssDNA might play only a minor role in the Tim-Tipin-RPA complex. (C) Hypothetical role of Tim-Tipin-RPA at the replication fork. During normal DNA replication Tim-Tipin could be bound to RPA adopting its 30 nt binding mode. After recruitment to the replication sites Tim-Tipin could get loaded onto DNA replication forks by a hand-off mechanism. During this process, RPA could perform a conformational switch adopting the 8 nt binding mode and thus releasing Tim-Tipin, which in turn is placed between the helicase and polymerase. Once placed at the fork, Tim-Tipin could then couple helicase-polymerase functions. In response to replicative stress or DNA damage accumulation of ssDNA occurs, which is covered by RPA. RPA could recruit Tim-Tipin to the accumulated ssDNA, which then becomes a part of the intra-S phase checkpoint protein complex and mediates efficient phosphorylation of Chk1 by ATR (6). Conformational change of RPA through phosphorylation (57) or interaction with other proteins could lead to Tim-Tipin release, which in turn resumes RPA-independent functions like DNA binding and helicase-polymerase coupling or associates with other forks.
