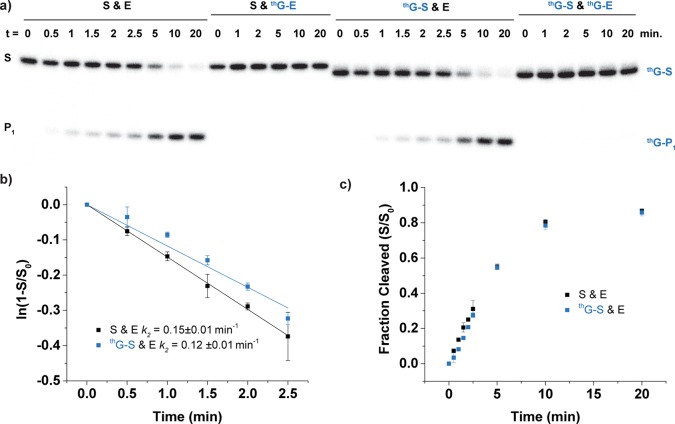
Figure 6.
(a) HH ribozyme cleavage reaction results were followed by 32P radioactive labeling of substrate strands S and thG-S. S and P1, and thG-S and thG-P1 indicate substrate and product strands (Figure 5). All reactions were conducted at 31 °C and contained 0.3 μM substrate (including a trace of 5′-32P labeled material), 3 μM enzyme, 50 mM Tris pH 7.0, 200 mM NaCl, and 10 mM MgCl2. The reactions were quenched at the given times (t in min) and resolved by gel electrophoresis on a denaturing 20% polyacrylamide gel with 7 M urea. (b) Initial kinetics of S and E and thG-S and E. The pseudo-first-order rate constants (k2) of the cleavage reactions are determined as the slope of ln(fraction cleaved) versus time. (c) Ribozyme-mediated cleavage curves as determined by 32P data for S and E and thG-S and E. Fraction cleaved (S/S0) was determined by dividing the amount of cleaved substrate by the sum of the full length and cleaved substrate.