Abstract
Background
Plant responses to phytohormone stimuli are the most important biological features for plants to survive in a complex environment. Cytokinin regulates growth and nutrient homeostasis, such as the phosphate (Pi) starvation response and Pi uptake in plants. However, the mechanisms underlying how cytokinin participates in Pi uptake and Pi signaling are largely unknown. In this study, we found that OsARF16 is required for the cytokinin response and is involved in the negative regulation of Pi uptake and Pi signaling by cytokinin.
Principal Findings
The mutant osarf16 showed an obvious resistance to exogenous cytokinin treatment and the expression level of the OsARF16 gene was considerably up-regulated by cytokinin. Cytokinin (6-BA) application suppressed Pi uptake and the Pi starvation response in wild-type Nipponbare (NIP) and all these responses were compromised in the osarf16 mutant. Our data showed that cytokinin inhibits the transport of Pi from the roots to the shoots and that OsARF16 is involved in this process. The Pi content in the osarf16 mutant was much higher than in NIP under 6-BA treatment. The expressions of PHOSPHATE TRANSPORTER1 (PHT1) genes, phosphate (Pi) starvation-induced (PSI) genes and purple PAPase genes were higher in the osarf16 mutant than in NIP under cytokinin treatment.
Conclusion
Our results revealed a new biological function for OsARF16 in the cytokinin-mediated inhibition of Pi uptake and Pi signaling in rice.
Introduction
The phytohormone, auxin, governs many aspects of growth, development and nutrient homeostasis in plants. Auxin perception and signal transduction regulate the expressions of many downstream genes via the AUXIN (AUX)/INDOLE-3ACETIC ACID (IAA) and AUXIN RESPONSE FACTOR (ARF)-mediated auxin signaling pathways [1]. ARFs, which regulate the expression of auxin-responsive genes, are involved in all the developmental processes from embryogenesis to senescence [2]. Many plant physiological functions depend on ARFs, including apical hook formation in seedlings [3], abaxial identity in the gynoecium [4], embryo development and vascular formation [5], hypocotyl tropisms [6], fruit development from fertilization [7], lateral root formation [8] and root system growth [9].
Auxin interacts with various phytohormones to regulate many physiological functions, either synergistically or antagonistically [10]–[13]. Among them, cytokinin is known to act antagonistically to auxin and plays an essential role in controlling plant morphogenesis [14]. Two primary cytokinin response transcription factors, ARR1 and ARR12, activate the SHORT HYPOCOTYL2 (SHY2) gene, which encodes a repressor that regulates the transcription of PIN-FORMED (PIN) auxin transporter genes [15]. Cytokinin inhibits the expression of auxin transport genes and prevents the establishment of an auxin gradient during lateral root initiation [16]–[18]. WOX11, a WUSCHEL-related homeobox gene, is induced by both auxin and cytokinin and represses a cytokinin-signaling negatively regulated gene, OsRR2 [19]. The mRNA accumulation of AtARF19, an auxin response factor involved in cell differentiation, has been shown to rely on the cytokinin-dependent transcription factor, AtARR12 [20]. Embryogenesis and post-embryonic root organ development all depend on the antagonistic interaction between cytokinin and auxin [21].
It has been shown that cytokinin is involved in the phosphate starvation response and phosphate uptake. The expressions of the phosphate transport gene, AtPT1, and the AtPHO1 family genes are suppressed by both auxin and cytokinin [22], [23]. Cytokinin also regulates many Pi deprivation-induced genes. For example, At4, which is expressed in the vascular tissue and is responsible for Pi accumulation in the shoots, is regulated by cytokinin [24]. AtIPS1 and other Pi starvation responsive genes are also repressed by exogenous cytokinin [25]. A global analysis of gene expression events in rice showed that the expression changes caused by Pi starvation were reversed by exogenous cytokinin treatment. A large increase in intracellular phosphate levels may reduce the phosphate starvation signaling triggered by exogenous cytokinin [26]. CRE1, the receptor for cytokinin, has been found to participate in suppressing the regulation of many genes following Pi deficiency [22], [27]. This implies that a close crosstalk exists between Pi and the cytokinin signaling transduction pathway [23].
Recently, it has been reported that OsARF12 was involved in Pi homeostasis and that OsARF16 facilitated the efficient utilization of Pi in rice [28], [29]. However, the underlying mechanism linking auxin/cytokinin and Pi signaling is largely unknown. In this study, we analyzed the differences in Pi transport and Pi signaling between the osarf16 mutant and NIP when subjected to cytokinin treatment. We found that OsARF16 was associated with cytokinin regulation of phosphate uptake and phosphate signaling in rice.
Materials and Methods
Plant materials and growth conditions
Rice plants (Oryza sativa L.) wild-type (WT) NIP and osarf16 mutant (a knock-out mutant) [29] were grown in culture solution combined with phytohormone treatments in a glasshouse with a light∶ dark cycle of 12∶ 12 h at 30∶ 24°C (day∶ night) and 60–70% humidity, and the pH was adjusted to 5.8 with HCl. For hydroponics, seedlings were transferred to a plastic net floating on the nutrient solution containing 0.323 mM NaH2PO4 or without NaH2PO4 for Pi deficiency treatment (−Pi or +Pi). Phytohormone treatments were performed with 0.01 µM to 1 µM of 6-BA for 7days, respectively. The hydroponic experiments were performed in a standard rice culture solution containing 1.425 mM NH4NO3, 0.323 mM NaH2PO4, 0.513 mM K2SO4, 0.998 mM CaCl2, 1.643 mM MgSO4, 0.009 mM MnCl2, 0.075 mM (NH4)6Mo7O24, 0.019 mM H3BO3, 0.155 mM CuSO4, 0.036 mM FeCl3, 0.070 mM citric acid and 0.152 mM ZnSO4.
β-glucuronidase (GUS) staining and analysis of GUS activity
The construction of OsARF16 promoter–GUS was performed according to a published method [29]. Exactly 2641 bp of the OsARF16 promoter sequence upstream of its ATG were cloned in front of the GUS gene and was introduced into the Agrobacterium tumefaciens strain EHA105. To examine the progression of cell division, the transgenic plant carrying pCYCB1;1:Dbox-GUS was crossed with osarf16 mutant and homozygous plants in the osarf16 background were assayed for GUS staining [30]. GUS staining of seedlings was performed using 100 mM sodium phosphate buffer (pH 7.0) containing 0.1% v/vTriton X-100 and 2 mM X-Gluc (Sangon, Shanghai, China), and samples were incubated at 37°C overnight. Stained tissues were observed using a Carl Zeiss laser scanning system LSM510 (http://www.zeiss.com/) and a Leica MZ95 stereomicroscope (Leica Instrument, Nusslosh, Germany). The measurement of GUS activity was performed as described by Jefferson [31].
QRT-PCR analysis
Total RNA was isolated from leaves or roots of 7-day-old seedlings. The methods for RNA extraction, reverse transcription and qRT-PCR were conducted as described in a previous report [29]. The sequences of the corresponding primers for qRT-PCR are listed in Table S1, S2, S3 and S4. OsACTIN was used as an internal standard to calculate the relative fold differences based on the comparative Ct method. 2−ΔΔCt refers to the fold difference in expression of cytokinin-related genes under cytokinin treatment (3 hr) compared with the untreated seedlings. Heat map representation was performed using the normalized Ct value with Treeview 1.6 to visualize the analysis data. The different colors correspond to the values of the gene change-fold ratio shown in the bar. The data were analyzed by three independent repeats.
Measurement of Pi contents
NIP and osarf16 mutants were analyzed to determine their total P contents. P measurements were performed using Flow Analyser SAN++ (Skalar Analytical B.V., Breda, Netherlands). For Pi transport experiment, the rice plants were grown for 7days under Pi deficiency solution, and then were transferred to Pi supply solution (1 mM Pi) with or without cytokinin (6-BA). Long time Pi starvation depressed total Pi level in rice seedlings and resulted in activity of Pi transport during the first 24 hour periods after Pi resupply. For each sample in the experiment, 0.1 g tissue was dried at 80°C for 48 hr and digested with HNO3/H2O2 at 110°C for 0.5 hr using a microware 3000 digestor (Anton Paar, Graz, Austria). Five biological replicates were performed for each sample in all experiments.
Statistics of root system parameters
The root system of rice plant was put into a container filled with distilled water. In order to minimize the intercross among the roots during image scanning, the whole root system was carefully separated into 5 portions, and each portion was transferred to individual container for scanning. The number of lateral roots was obtained by scanning. The average lengths of root hairs (3 mm from the root tips) were measured by a LEICA MZ95 stereomicroscope with camera scale (Leica Instrument, Nusslosh, Germany).
Qualitative analysis of root-associated APase activity
Root APase staining was performed according to Bozzo's report [32]. The roots were excised from 7 days Pi-deprived and Pi-supplied seedlings and incubated with a 5-bromo-4-chloro-3-indolyl-phosphate (BCIP) content agar solution containing 50 mM sodium acetate (pH 5.5) with 10 mM MgCl2, 0.6% agar and 0.08% BCIP at room temperature for 20 min. The blue color on the root surface, formed by hydrolysis of BCIP, was photographed using an EOS 40D camera (Canon Corporation, Tokyo, Japan). Protein (1 µg) was used for APase activity assay, and the protein was added to 620 µl of reaction buffer (50 mM NaAc pH 5.5 and 10 mM MgCl2), and 10 µl of p-nitrophenol phosphate (10 mg ml−1 pNPP; Sigma). After incubation at 37°C for 10 min, the reaction was stopped by 1.2 ml of 1M NaOH, and then absorbance was measured at 412 nm wavelength. Phosphatase activity was counted as ng of pNPP accumulated µg−1 soluble protein min−1. These experiments were repeated three times.
Results
Physiological and morphological evidence that OsARF16 is involved in cytokinin responses in rice seedlings
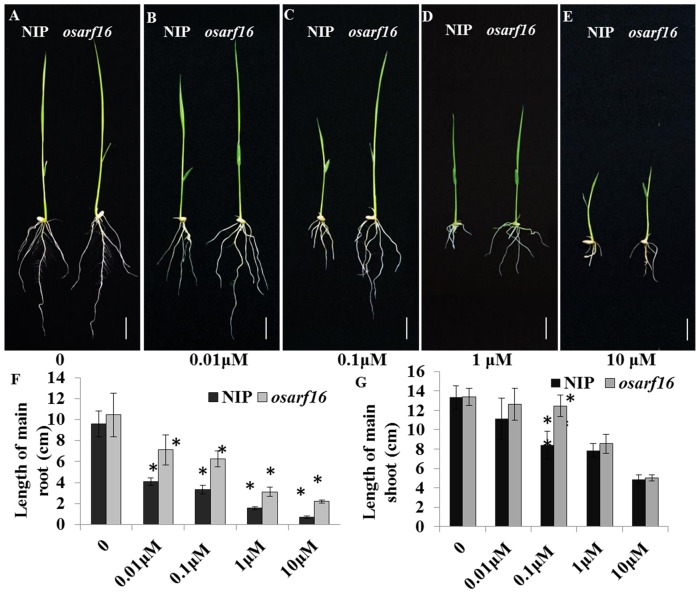
In previous studies, we have reported that the OsARF16 is required for auxin signaling and the phosphate starvation response in rice. The phenotype of osarf16 mutant under Pi deficiency condition was well studied in our last publication [29]. In the present study, the mutant osarf16 was used to investigate how auxin response factor is involved in cytokinin signaling. To explore the possible effects of OsARF16 on morphological and physiological responses to cytokinin (6-BA) treatment, wild-type (NIP) and mutant osarf16 seeds were grown in nutrient solutions containing different concentrations of 6-BA. The concentrations of 6-BA used in this experiment were 0, 0.01, 0.1, 1.0 and 10 µM. After 7 days of treatment, the root growth was considerably inhibited by 6-BA in the NIP seedlings (Fig. 1A–E). The root lengths of NIP declined significantly from 9.59 cm to 0.72 cm when the 6-BA concentration increased from 0 to 10 µM. Under the same conditions, the root growth inhibition was less significant in the osarf16 mutant. As the 6-BA concentration increased, the osarf16 mutant root lengths fell from 10.44 cm to 2.21 cm (Fig. 1F). In addition to root growth inhibition, shoot elongation was also affected by 6-BA application in NIP. The shoot lengths of NIP declined significantly from 13.32 cm to 4.87 cm when the 6-BA concentration increased from 0 to 10 µM. Under the same conditions, the root growth inhibition was less significant in the osarf16 mutant. Specially, the shoot length in NIP was only 68% of that in the osarf16 mutant under the 0.1 µM 6-BA treatment (Fig. 1C, G).
Figure 1. Physiological evidences for involvement of OsARF16 in cytokinin (6-BA) Responses.
(A–E) Phenotype of NIP and osarf16 mutant under different concentration of 6-BA treatments (0/0.01/0.1/1/10 µM) using 7-day-old seedlings (Bar represents 2 cm). The graphs represent statistics data of main root length (F) and main shoot length (G). Data are shown as the mean ± SD (n = 5). Significant (P<0.05) differences in length of main root and main shoot between osarf16 and NIP are indicated by an asterisk.
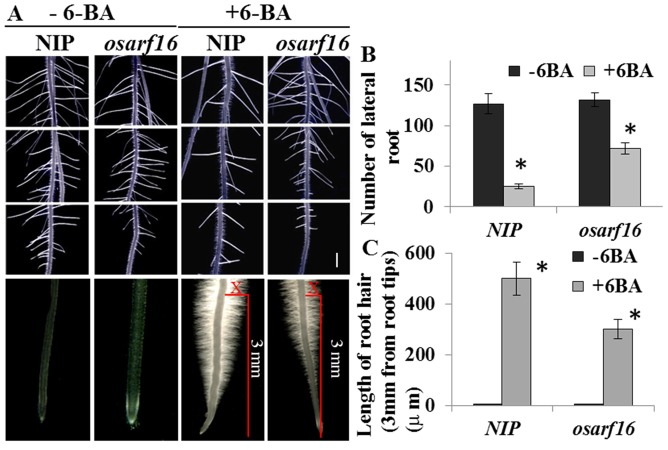
Cytokinin regulates root system architecture (RSA) by disturbing cell division activity and increasing the length of the root hairs at the root tips [33], [34]. In the present study, we measured the number of lateral roots on the main roots and the average lengths of the root hairs on the root tips of both NIP and the osarf16 mutant under the 0.1 µM 6-BA treatment. The results showed that the number of lateral roots on osarf16 was much greater than on NIP under 0.1 µM 6-BA treatment (Fig. 2A). Without 6-BA application, the number of lateral roots on NIP was similar to the osarf16 mutant. The 0.1 µM 6-BA treatment reduced the number of lateral roots by 80% in the NIP seedlings. However, the reduction was only 45% in the osarf16 mutant seedlings (Fig. 2B). The number of lateral roots in osarf16 primary root was almost twice as much as NIP under 0.1 µM 6-BA treatment. When 6-BA was not applied, there were no root hairs on the root tips of both NIP and the osarf16 mutant. On average, the lengths of the root hairs (3 mm from the root tips) could be increased to almost 500 µm on the tips of NIP, but only to 300 µm on the tips of the osarf16 mutant under the 0.1 µM 6-BA treatment (Fig. 2C). The results revealed that the osarf16 mutant was more insensitive to cytokinin treatment than NIP.
Figure 2. Effects of cytokinin on regulation of root system architecture (RSA).
(A) 0.1 µM 6-BA treatment reduced the number of lateral root in primary root and induced root hair of NIP and osarf16 mutant in primary root tips (The bar represents 5 mm). The graphs represent statistics data of lateral root number (B) and average root hairs length (C). Data are shown as the mean ± SD (n = 5). Significant (P<0.05) differences in number of lateral root in primary root and average length of root hairs in primary root tips between osarf16 and NIP are indicated by an asterisk.
To confirm that the phenotypes described above were caused by the loss-of-function of OsARF16, we transformed the OsARF16 gene back into the osarf16 mutant and three independent complementation lines (osarf16/C) were chosen for analysis. The growth parameters for NIP and osarf16/C were approximately the same under the different 6-BA treatments (0, 0.01, 0.1, 1.0 and 10 µm) (Figure S1). Again, we used various cytokinins (kinetin, zeatin) to confirm the involvement of OsARF16 in the cytokinin responses. Kinetin and zeatin inhibited root system elongation and shoot growth and osarf16 was, again, less sensitive to both cytokinins than NIP (Figure S2). The results confirmed that OsARF16 was involved in plant responses to cytokinin.
OsARF16 affects cytokinin signaling
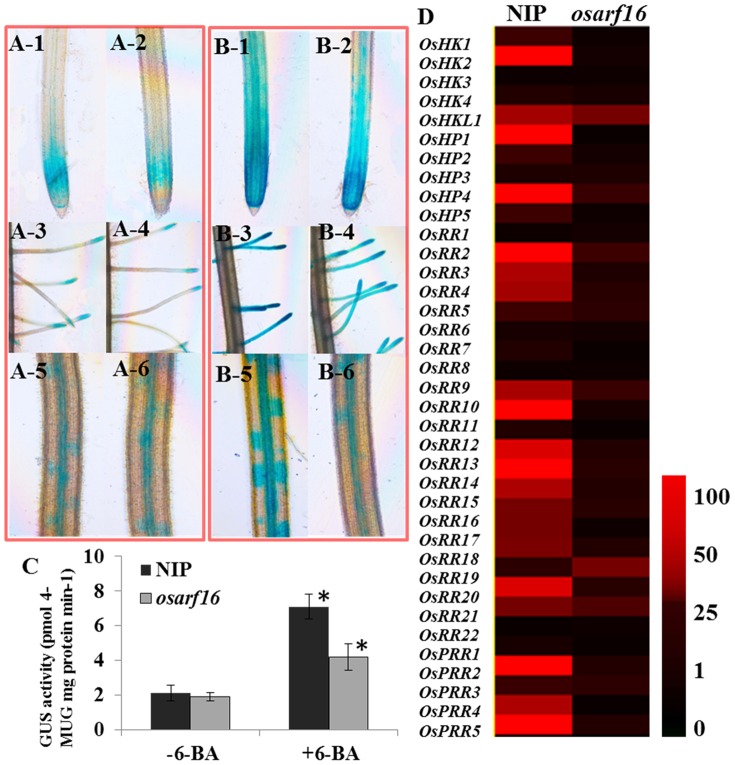
To further determine the cytokinin signaling changes in the osarf16 mutant, we analyzed cell division activity in the primary root tips, the lateral roots in primary root and the lateral root primordia under 6-BA treatment using the pCYCB1;1:Dbox-GUS reporter [30]. Five positive transgenic lines were used for this analysis. Under the control treatment, the cell division activities, as indicated by the expressions of the pCYCB1;1:Dbox-GUS reporter in NIP and the osarf16 mutant, were almost the same. After 6-BA treatment (3 hr), the cell division activities in the primary root tips, the lateral roots in primary root and the lateral root primordia considerably increased in NIP, whereas the induction of cell division activity in osarf16 was much lower than in NIP (Fig. 3A, B). The GUS activity data is shown in Fig. 3C. The GUS activity under −6-BA treatment in NIP was almost the same as that in osarf16 mutant. However, the GUS activity in NIP was 69.0% higher than that in osarf16 mutant. The data was essentially in agreement with the GUS staining results showed in Fig. 3 A and B.
Figure 3. OsARF16 affects cytokinin signaling.
(A, B) pCYCB1;1:Dbox-GUS staining was performed in primary root tips, lateral roots and lateral root primordia of NIP and osarf16 mutant under −6-BA/+6-BA treatment. Concentration of 6-BA in the treatments was 0.1 µM. pCYCB1;1:Dbox-GUS staining in root tips of NIP (A-1) and osarf16 mutant (A-2) under −6-BA treatment; pCYCB1;1:Dbox-GUS staining in lateral roots of NIP (A-3) and osarf16 mutant (A-4) under −6-BA treatment; pCYCB1;1:Dbox-GUS staining in lateral root primordia of NIP (A-5) and osarf16 mutant (A-6) under −6-BA treatment; pCYCB1;1:Dbox-GUS staining in primary root tips of NIP (B-1) and osarf16 mutant (B-2) under +6-BA treatment; pCYCB1;1:Dbox-GUS staining in lateral roots of NIP (B-3) and osarf16 mutant(B-4) under +6-BA treatment; pCYCB1;1:Dbox-GUS staining in lateral root primordia of NIP (B-5) and osarf16 mutant (B-6) under +6-BA treatment. (C) Analysis of GUS activity. The entire root of the seedling was used for analysis. The graph represents statistics data of GUS activity in NIP and osarf16 mutant roots. Data are shown as the mean ± SD (n = 5). Significant (P<0.05) differences in GUS activity between osarf16 and NIP are indicated by an asterisk. (D) Analysis of the expression levels of cytokinin signaling related genes in osarf16 mutant and NIP. The data of 2−ΔΔCt (qRT data) refers to the fold difference in expression of cytokinin-related genes under cytokinin treatment (3 hr) compared with the untreated seedlings. Heat map representation was performed using the normalized 2−ΔΔCt values with Treeview 1.6 to visualize the analysis data. The different colors correspond to the values of the gene change-fold ratio shown in the bar. The data were analyzed by three independent repeats.
Many studies have investigated cytokinin signaling in rice [35], [36]. We analyzed the expression of 37 cytokinin response genes in both NIP and the osarf16 mutant in order to improve our understanding of the mechanism behind OsARF16 involvement in the cytokinin signaling system. These genes are all part of two-component regulatory systems. Five belong to HKs (cytokinin-response histidine protein kinase) (OsHK1–4, OsHKL1), five to HPs (histidine phosphotransfer proteins) (OsHP1–5), fifteen to type-A RRs (response regulators) (OsRR1–15), seven to type-B RR genes (OsRR16–22), and five are predicted pseudo-response regulators (OsPRR1–5) [37]. A qRT-PCR analysis was performed on the roots of 7-day-old NIP and osarf16 seedlings grown in culture solutions with or without 6-BA (the primer sequences information is provided in Table S1). The results showed that all 37 cytokinin responsive genes were sharply induced by 6-BA treatment in the roots of the NIP seedlings. However, in the osarf16 mutant seedlings, the induction of these cytokinin responsive genes was reduced under the 6-BA treatment compared to the NIP seedlings (Fig. 3D). This suggested that cytokinin signaling in osarf16 was compromised compared to NIP.
The expression pattern of OsARF16 was dramatically changed by cytokinin treatment
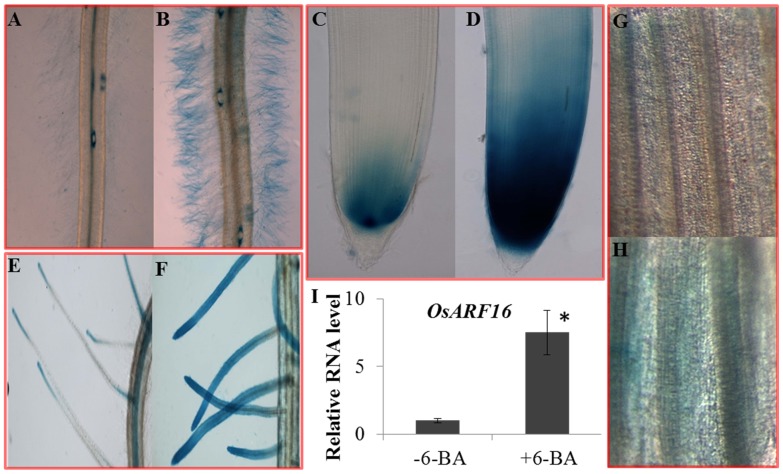
We used the GUS reporter gene to evaluate the OsARF16 expression patterns in the roots and shoots under −6-BA treatment and +6-BA treatment conditions. Five positive transgenic lines were used for this analysis. Under the control treatment (−6-BA treatment), OsARF16 was expressed in root tips and stele of both primary roots (Fig. 4 A, C) and lateral roots in primary root (Fig. 4 E). Under +6-BA treatment (3 hr), the OsARF16 expression level increased several times compared to the control treatment, especially in the root hairs (Fig. 4A–F). The OsARF16 expression level was almost undetectable in root hairs under the control treatment, but was strongly induced by the +6-BA treatment. Meanwhile, the expression level of OsARF16 was also induced by 6-BA treatment in shoots (Fig. 4G, H). We used qRT-PCR to confirm the OsARF16 expression pattern changes in roots under the control and +6-BA treatment conditions and the results were consistent with the GUS staining analysis results (Fig. 4I). The expression level was 7.5 folds induced by 6-BA treatment in the whole root organ.
Figure 4. Analysis of OsARF16 expression pattern under cytokinin treatment using OsARF16-Promoter: GUS lines.
The expression pattern of OsARF16 in primary root tips under (A) control and (B) 0.1 µM 6-BA treatment for 3 hr; The expression pattern of OsARF16 in root hairs of primary root under (C) control and (D) 0.1 µM 6-BA treatment for 3 hr; The expression pattern of OsARF16 in lateral roots of primary root under (E) control and (F) 0.1 µM 6-BA treatment for 3 hr. The expression pattern of OsARF16 in leaves under (G) control and (H) 0.1 µM 6-BA treatment for 3 hr. (I) qRT-PCR method was used to confirm the OsARF16 expression pattern changes in roots under 0.1 µM 6-BA treatment for 3 hr. Significant (P<0.05) differences in the expression level of OsARF16 between 6-BA treatment and mock treatment is indicated by an asterisk.
Analysis of total phosphorus (P) content over a time-course in NIP and the osarf16 mutant
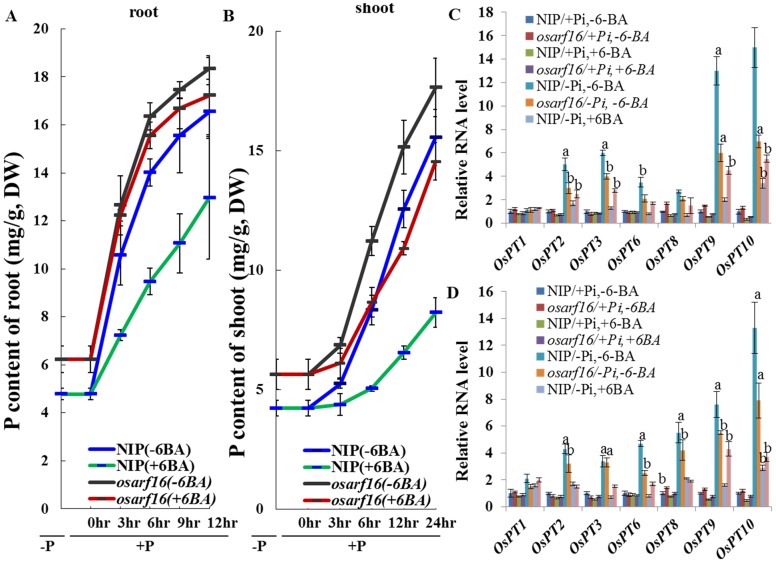
The total P contents over a time-course were measured in NIP and the osarf16 mutant seedlings in order to clarify the function of OsARF16 in the cytokinin-mediated inhibition of phosphate transport between roots and shoots. The Pi-deprived rice seedlings, which were grown in a Pi free nutrient solution for 7 days, were transferred to a nutrient solution containing 1 mM Pi in order to monitor P contents in the shoots over a 24 h period and in roots over a 12 h period after Pi application. After 7 days of Pi starvation, the total P contents were only 4.7 mg g−1 in the NIP roots and 6.2 mg g−1 in the osarf16 mutant roots. When resupplied with 1 mM Pi, the P contents in the roots rapidly increased in both NIP (16.55 mg g−1) and osarf16 (18.35 mg g−1) after 12 h of Pi resupply. Under the same conditions, the P contents in the shoots were 4.2 mg g−1 in NIP and 5.6 mg g−1 in the osarf16 mutant. When resupplied with 1 mM Pi, the P content only increased to 12.96 mg g−1 in NIP roots with 6-BA application (78.3% of the 6-BA free treatment) after 24 h of Pi resupply and increased to 17.23 mg g−1 in the osarf16 shoots (93.4% of the 6-BA free treatment). On the other hand, the P contents in the shoots also rose in both NIP (15.56 mg g−1) and osarf16 (17.65 mg g−1) after 24 h of Pi resupply. With 6-BA application, the P content only increased to 8.2 mg g−1 in NIP shoots (52.9% of the 6-BA free treatment) after 24 h of Pi resupply and increased to 14.5 mg g−1 in the osarf16 shoots (82.9% of the 6-BA free treatment). Cytokinin (6-BA) clearly inhibited Pi transport in NIP, but the inhibition of Pi transport by cytokinin was less obvious in the osarf16 mutant seedlings compared to NIP (Fig. 5A, B).
Figure 5. Analysis of total phosphorus (P) contents over a time-course in both NIP and osarf16 mutant.
(A,B) Total P contents in NIP and osarf16 mutant after Pi resupply using 7-day-old Pi-deprived seedlings: (A) in roots and (B) in shoots. The statistics of P contents were calculated for five independent biological replications. (C, D) Expression of Pi transporter coding genes in Pi-sufficient (+Pi) and Pi-deficient (−Pi) treatments with or without 6-BA (C) in roots and (D) in shoots. Concentration of 6-BA in the treatments was 0.1 µM. The ACTIN gene of rice was used as the reference gene for qRT-PCR. Value ± SD of five independent replicates. “a” indicated significant difference in expression levels of OsARF16 from treatments to mock at 1% by student's t test. “b” indicated significant difference in expression levels of OsARF16 from treatments to mock at 5% by student's t test.
Furthermore, we analyze shoot/root ratios of P contents in both NIP and osarf16 mutant under different conditions. After 7 days Pi starvation, the shoot/root ratios of P contents in both NIP and osarf16 mutant were nearly 50%. When resupplied with 1 mM Pi, the shoot/root ratios of P contents were increased quickly after 3 hours Pi resupply and decreased to 50% gradually thereafter in both NIP and osarf16 mutant. With 6-BA application, the shoot/root ratios of P contents increased to 62% after 3 hours Pi resupply and did not decreased obviously during 12 hour Pi resupply period in NIP. With 6-BA application, the shoot/root ratios of P contents increased to 65% after 3 hours Pi resupply and decreased obviously during 12 hour Pi resupply period in osarf16 mutant (Figure S3). The 6-BA treatment affects the shoot/root ratios of P contents in NIP seedlings, but it was less obvious in the osarf16 mutant.
Involvement of OsARF16 in cytokinin-mediated inhibition of Pi transport
The PHOSPHATE TRANSPORTER1 (PHT1) gene family plays a number of critical roles in Pi uptake, translocation and homeostasis in Arabidopsis. Some of these genes have also been identified in rice [38], [39]. In this study, we used qRT-PCR to analyze the expression levels of several members of the rice PHT1 gene family (including OsPT1/2/3/6/8/9 and 10). The primer sequences information is provided in Table S2. Most of these phosphate transporter coding genes were up-regulated by Pi deficiency and their expression levels were inhibited in NIP roots and shoots by applications of 0.1 µM 6-BA (Fig. 5C, D). The expression levels of these OsPTs were higher in the osarf16 mutant than in NIP under −Pi/+6-BA treatments.
Expression analysis of phosphate (Pi) starvation-induced (PSI) genes in NIP and the osarf16 mutant
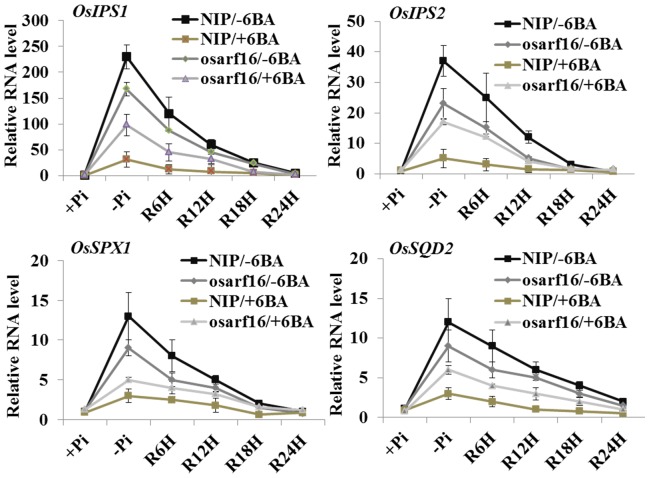
To test whether the phosphate (Pi) starvation-induced (PSI) genes were also affected by cytokinin treatment, we chose four genes: OsIPS1/OsIPS2, OsSPX1 [40] and OsSQD2 [41]. Then we analyzed the transcript abundance in NIP and the osarf16 mutant and under +Pi/−6-BA, −Pi/−6-BA, +Pi (resupply)/−6-BA, +Pi/+6-BA, −Pi/+6-BA and +Pi (resupply)/+6-BA treatments (The primer sequences information are provided in Table S3). The expression level analysis revealed that these four genes were sharply up-regulated under the −Pi/−6-BA treatment and quickly fell down to background levels during the 24 h period after Pi resupply (Fig. 6). All four PSI genes were repressed by 6-BA application under the −Pi treatment in NIP. For example, OsIPS1 was 230 times higher under the −Pi/−6-BA treatment compared to the +Pi/−6-BA treatment, but was only 25 times higher under −Pi/+6-BA treatment. In the osarf16 mutant, the expression levels of these PSI genes were much higher than that in NIP under the −Pi/+6-BA treatment.
Figure 6. Expression analysis of phosphate (Pi) starvation-induced (PSI) genes, OsIPS1, OsIPS2, OsSPX1, OsSQD2 in NIP and osarf16 mutant.
Analysis of expressions were performed under phosphate-sufficient (+Pi), phosphate-deficient (−Pi) and Pi resupply conditions (R6h, R12h, R18h and R24h refer to resupply times of 6, 12, 18 and 24 h, respectively) with or without 6-BA treatments. Concentration of 6-BA in the treatments was 0.1 µM. 7-day-old seedlings of NIP and osarf16 mutant were used for quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis. Value ± SD of five independent replicates.
Analysis of acid phosphatase (APase) activity and expression of six PAPase genes
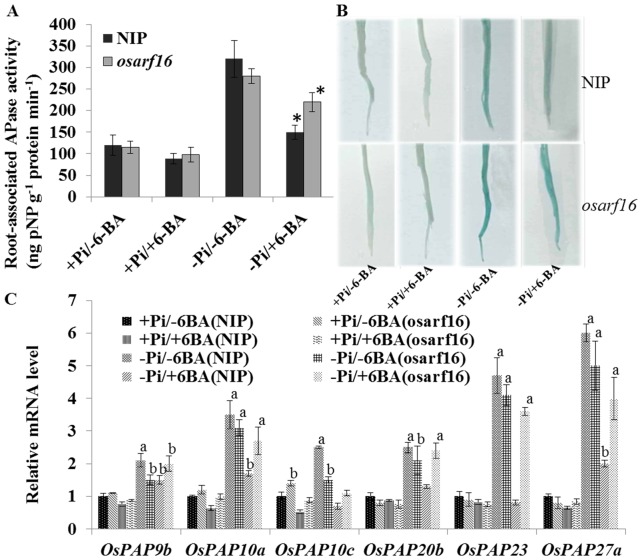
The induction of APase activity under Pi deficiency conditions helps to catalyze inorganic phosphate hydrolysis from organophosphates and is an important strategy that enables plants to deal with Pi deficiency [42]. The expressions of PAPase genes and APase activity were measured in NIP and the osarf16 mutant in order to understand how OsARF16 is involved in Pi homeostasis through its effects on APase activity. Rice seedlings were grown under +Pi/−6BA, +Pi/+6BA, −Pi/−6BA and −Pi/+6BA conditions. In contrast, 6-BA application significantly suppressed the APase activity in NIP under Pi deficiency conditions (−Pi/+6-BA treatment). However, the APase activity was 50% higher in the osarf16 mutant than in NIP the under −Pi/+6-BA treatment (Fig. 7A). The data were gained from the analysis of root intracellular APase activity on substrate pNPP (Fig. 7B). Many purple APase (PAPase) genes, which are involved in Pi uptake and Pi starvation responses, have been reported by Wang et al. (2009). We chose six of the PAPase genes for this experiment (OsPAP9b, OsPAP10a, OsPAP10c, OsPAP20b, OsPAP23 and OsPAP27a) because they were induced by −Pi deficiency by more than two-fold (The primer sequences information is provided in Table S4). Notably, all six genes were down-regulated by 6-BA treatment under the −Pi deficiency treatment in NIP. The expression levels of these genes were higher in the osarf16 mutant than in NIP under the −Pi/+6-BA treatment (Fig. 7C). This suggested that cytokinin-mediated inhibition of APase expression is dependent on OsARF16.
Figure 7. Analysis of acid phosphatase (APase) activity and expression of six purple APase coding genes.
(A) Acid phosphatase (APase) activity in NIP and osarf16 mutant under +Pi/−6BA, +Pi/+6BA, −Pi/−6BA and −Pi/+6BA conditions respectively. Concentration of 6-BA in the treatments was 0.1 µM. The graph represents statistics data of root-associated APase activity. (B) Acid phosphatase (APase) activity in the root surface of NIP and osarf16 mutant under +Pi/−6BA, +Pi/+6BA, −Pi/−6BA and −Pi/+6BA conditions respectively. (C) Expression levels of six purple APase coding genes under +Pi/−6BA, +Pi/+6BA, −Pi/−6BA and −Pi/+6BA conditions respectively. Data are shown as the mean ± SD (n = 5). Significant (P<0.05) differences in APase activity between osarf16 mutant and NIP are indicated by an asterisk. “a” indicated significant difference in expression levels of OsPAPs from treatments to mock at 1% by student's t test. “b” indicated significant difference in expression levels of OsPAPs from treatments to mock at 5% by student's t test.
Discussion
OsARF16 is a key regulator of the cytokinin response in rice
ARFs are involved in many important biological functions, including plant development and nutritional homeostasis [8], [43]. OsARF16 as a transcriptional activator that is highly homologous to AtARF7/19 in Arabidopsis and is implicated in Pi deficiency response related root system architecture changes [29], [44]. In Arabidopsis meristems, cytokinin controls the differentiation rate of transit-amplifying cells by antagonizing the plant hormone auxin, which is needed to sustain cell proliferation [15]. In our previous studies, we identified the osarf16 mutant and characterized its biological effects on auxin signaling and auxin distribution [29]. However, how ARFs interact with cytokinin signaling in rice is largely unknown.
Cytokinin suppresses the growth of rice seedlings and OsARF16 loss-of-function attenuates sensitivity to exogenous cytokinin treatment (Fig. 1). High concentration 6-BA treatments cause severe growth defects in wild-type seedlings, including root system development and shoot elongation. In particular, there was an induction in lateral root numbers and main root growth in the osarf16 mutant compared to NIP under 6-BA treatment. This suggested that OsARF16 was required for cytokinin responses in rice.
In rice plants, PINs, and LAXs are the two major gene families responsible for auxin transport in vivo [29]. Exogenous cytokinin treatment also inhibited the expression of auxin transport genes and disrupted auxin distribution, which is required for many of the physiological responses to cytokinin [45]. Our data showed that 6-BA treatment clearly down-regulated the expression levels of OsPINs and OsLAXs in NIP. However, the expression levels of the OsPIN and OsLAX family genes were much higher in the osarf16 mutant than in NIP under the 6-BA treatment (Figure S4). OsARF16 may be involved in cytokinin responses by regulating the expression of auxin transporter coding genes.
OsARF16 is involved in cytokinin-mediated negative regulation of phosphate uptake and phosphate signaling in rice (Oryza sativa L.)
Cytokinin inhibits phosphate transport by regulating the expression of the phosphate transporter coding genes [22], [23]. In this study we used 7-day-old Pi-deprived seedlings to investigate how OsARF16 is involved in the negative regulation of Pi uptake and transport by cytokinin. When transferred to the Pi supply nutrient solution, the phosphate contents in NIP seedlings rapidly rose (Fig. 5A) and cytokinin application could significantly block this process. However, in the osarf16 mutant, the effect of cytokinin on preventing Pi transport from the roots to the shoots was weaker than in NIP (Fig. 5B). The expression levels of OsPTs were much higher in the osarf16 mutant than that in NIP under the 6-BA treatment, which was consistent with the results of the Pi content measurements. These results indicated that OsARF16 was involved in cytokinin-mediated inhibition of Pi transport from the roots to the shoots by regulating PT expressions in rice.
OsARF16 is an important transcription factor involved in the crosstalk between cytokinin and Pi starvation signaling
It has been already reported that Pi deficiency signaling could be inhibited by cytokinin and that there was a crosstalk between cytokinin and Pi deficiency signaling [25]. Cytokinin may repress the Pi starvation response by increasing intracellular phosphate content. Under Pi deficiency condition, more organic Pi is released into cellular Pi pool (inorganic Pi) driven by exogenous 6-BA [26]. In our experiment, total Pi (organic Pi and inorganic Pi) have been measured in NIP and osarf16 mutant under different conditions. After 7 days Pi starvation, the Pi level in rice seedlings was greatly depressed and the increased of total Pi content after Pi resupply was inhibited by 6-BA treatment (Fig. 5). 6-BA treatment elevates inorganic Pi content by reducing organic Pi concentration (major component of total Pi). However, the full physiological and molecular relevance of how cytokinin is involved in Pi signaling remains unclear. In this study, we discovered that OsARF16, an auxin response factor, was an important regulator involved in the crosstalk between cytokinin and Pi deficiency signaling. Pi signaling was repressed by cytokinin treatment and it depended on a functioning OsARF16 gene. Under Pi deficiency conditions, the expressions of OsIPS1/2, OsSPX1 and OsSQD2 were inhibited by 6-BA treatment in NIP. However, inhibition of PSI gene expressions by 6-BA treatment in the osarf16 mutant was compromised compared to the NIP seedlings.
Acid phosphatase activity was also reduced by cytokinin treatment. An increase in APase activity in the roots is a typical Pi starvation response when plants are subjected to Pi deficiency. Induction and secretion of acid phosphatases (APases) is a highly efficient mechanism that helps plants to survive and grow under Pi deficient conditions [28], [46], [47]. According to the results (Fig. 7A, B), the APase activity in NIP roots was reduced by 6-BA treatment under Pi deficiency conditions. The APase activity was much stronger in the osarf16 mutant roots compared to the NIP roots under the same conditions. The expression level of some purple APase coding genes, such as OsPAP20b, 23 and 27a in the osarf16 mutant under the −Pi/+6-BA treatment, were almost twice than in NIP. These results strongly demonstrated that OsARF16 is involved in the crosstalk between cytokinin and Pi starvation signaling.
Conclusion
In summary, we found that OsARF16 was involved in cytokinin-mediated inhibition of phosphate transport and phosphate deficiency signaling in rice. Our data show that OsARF16 plays a critical role in auxin and Pi starvation responses and is involved in the crosstalk between cytokinin and Pi deficiency signaling. Our next study will focus on the direct downstream targets of OsARF16 in order to reveal how cytokinin regulates Pi transport and Pi signaling in rice.
Supporting Information
Physiological results for osarf16/C under 6-BA treatment.
(DOCX)
Physiological evidence for OsARF16 was involved in various cytokinins (kinetin, zeatin) Responses.
(DOCX)
Analysis of shoot/root ratios of P contents in both NIP and osarf16 mutant.
(DOCX)
The expression levels of OsPIN and OsLAX family genes.
(DOCX)
The RT primer sequences 37 cytokinin response genes.
(DOCX)
Primer sequences for phosphorus transporters.
(DOCX)
Primer sequences for the Phosphate Starvation Induced genes ( PSIs ).
(DOCX)
Primer sequences for purple acid phosphatase genes.
(DOCX)
Acknowledgments
We are grateful to Dr. Yanhua Qi (College of Life Sciences, Zhejiang University) for the kind gifts of osarf16 mutant and OsARF16 promoter: GUS transgenic lines, to Dr. Dean Jiang for technical support.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded by the National Natural Science Foundation of China (31401935) and Zhejiang Provincial Natural Science Foundation of China under Grant No. LQ14C060001. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Mockaitis K, Estelle M (2008) Auxin receptors and plant development: a new signaling paradigm. Annu Rev Cell Dev Biol 24: 55–80. [DOI] [PubMed] [Google Scholar]
- 2. Guilfoyle TJ, Hagen G (2007) Auxin response factors. Curr Opin Plant Biol 10: 453–460. [DOI] [PubMed] [Google Scholar]
- 3. Schruff MC, Spielman M, Tiwari S, Adams S, Fenby N, et al. (2006) The AUXIN RESPONSE FACTOR 2 gene of Arabidopsis links auxin signalling, cell division, and the size of seeds and other organs. Development 133: 251–261. [DOI] [PubMed] [Google Scholar]
- 4. Sessions A, Nemhauser JL, McColl A, Roe JL, Feldmann KA, et al. (1997) ETTIN patterns the Arabidopsis floral meristem and reproductive organs. Development 124: 4481–4491. [DOI] [PubMed] [Google Scholar]
- 5. Hardtke CS, Berleth T (1998) The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J 17: 1405–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li J, Dai X, Zhao Y (2006) A role for auxin response factor 19 in auxin and ethylene signaling in Arabidopsis. Plant Physiol 140: 899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goetz M, Vivian-Smith A, Johnson SD, Koltunow AM (2006) AUXIN RESPONSE FACTOR8 is a negative regulator of fruit initiation in Arabidopsis. Plant Cell 18: 1873–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Smet I, Lau S, Voss U, Vanneste S, Benjamins R, et al. (2010) Bimodular auxin response controls organogenesis in Arabidopsis. Proc Natl Acad Sci U S A 107: 2705–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu B, Li YH, Wu JY, Chen QZ, Huang X, et al. (2011) Over-expression of mango (Mangifera indica L.) MiARF2 inhibits root and hypocotyl growth of Arabidopsis. Mol Biol Rep 38: 3189–3194. [DOI] [PubMed] [Google Scholar]
- 10. Agusti J, Herold S, Schwarz M, Sanchez P, Ljung K, et al. (2011) Strigolactone signaling is required for auxin-dependent stimulation of secondary growth in plants. Proc Natl Acad Sci U S A 108: 20242–20247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sakamoto T, Morinaka Y, Inukai Y, Kitano H, Fujioka S (2013) Auxin signal transcription factor regulates expression of the brassinosteroid receptor gene in rice. Plant J 73: 676–688. [DOI] [PubMed] [Google Scholar]
- 12. Vandenbussche F, Callebert P, Zadnikova P, Benkova E, Van Der Straeten D (2013) Brassinosteroid control of shoot gravitropism interacts with ethylene and depends on auxin signaling components. Am J Bot 100: 215–225. [DOI] [PubMed] [Google Scholar]
- 13. Yang DL, Yang Y, He Z (2013) Roles of plant hormones and their interplay in rice immunity. Mol Plant 6: 675–685. [DOI] [PubMed] [Google Scholar]
- 14. Bielach A, Duclercq J, Marhavy P, Benkova E (2012) Genetic approach towards the identification of auxin-cytokinin crosstalk components involved in root development. Philos Trans R Soc Lond B Biol Sci 367: 1469–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dello Ioio R, Nakamura K, Moubayidin L, Perilli S, Taniguchi M, et al. (2008) A genetic framework for the control of cell division and differentiation in the root meristem. Science 322: 1380–1384. [DOI] [PubMed] [Google Scholar]
- 16. Marhavy P, Duclercq J, Weller B, Feraru E, Bielach A, et al. (2014) Cytokinin controls polarity of PIN1-dependent auxin transport during lateral root organogenesis. Curr Biol 24: 1031–1037. [DOI] [PubMed] [Google Scholar]
- 17. Laplaze L, Benkova E, Casimiro I, Maes L, Vanneste S, et al. (2007) Cytokinins act directly on lateral root founder cells to inhibit root initiation. Plant Cell 19: 3889–3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marhavy P, Bielach A, Abas L, Abuzeineh A, Duclercq J, et al. (2011) Cytokinin modulates endocytic trafficking of PIN1 auxin efflux carrier to control plant organogenesis. Dev Cell 21: 796–804. [DOI] [PubMed] [Google Scholar]
- 19. Kitomi Y, Ito H, Hobo T, Aya K, Kitano H, et al. (2011) The auxin responsive AP2/ERF transcription factor CROWN ROOTLESS5 is involved in crown root initiation in rice through the induction of OsRR1, a type-A response regulator of cytokinin signaling. Plant J 67: 472–484. [DOI] [PubMed] [Google Scholar]
- 20. Perilli S, Perez-Perez JM, Di Mambro R, Peris CL, Diaz-Trivino S, et al. (2013) RETINOBLASTOMA-RELATED protein stimulates cell differentiation in the Arabidopsis root meristem by interacting with cytokinin signaling. Plant Cell 25: 4469–4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Muller B, Sheen J (2008) Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature 453: 1094–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Karthikeyan AS, Varadarajan DK, Mukatira UT, D'Urzo MP, Damsz B, et al. (2002) Regulated expression of Arabidopsis phosphate transporters. Plant Physiol 130: 221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ribot C, Wang Y, Poirier Y (2008) Expression analyses of three members of the AtPHO1 family reveal differential interactions between signaling pathways involved in phosphate deficiency and the responses to auxin, cytokinin, and abscisic acid. Planta 227: 1025–1036. [DOI] [PubMed] [Google Scholar]
- 24. Shin H, Shin HS, Chen R, Harrison MJ (2006) Loss of At4 function impacts phosphate distribution between the roots and the shoots during phosphate starvation. Plant J 45: 712–726. [DOI] [PubMed] [Google Scholar]
- 25. Martin AC, del Pozo JC, Iglesias J, Rubio V, Solano R, et al. (2000) Influence of cytokinins on the expression of phosphate starvation responsive genes in Arabidopsis. Plant J 24: 559–567. [DOI] [PubMed] [Google Scholar]
- 26. Wang X, Yi K, Tao Y, Wang F, Wu Z, et al. (2006) Cytokinin represses phosphate-starvation response through increasing of intracellular phosphate level. Plant Cell Environ 29: 1924–1935. [DOI] [PubMed] [Google Scholar]
- 27. Franco-Zorrilla JM, Martin AC, Solano R, Rubio V, Leyva A, et al. (2002) Mutations at CRE1 impair cytokinin-induced repression of phosphate starvation responses in Arabidopsis. Plant J 32: 353–360. [DOI] [PubMed] [Google Scholar]
- 28. Wang S, Zhang S, Sun C, Xu Y, Chen Y, et al. (2014) Auxin response factor (OsARF12), a novel regulator for phosphate homeostasis in rice (Oryza sativa). New Phytol 201: 91–103. [DOI] [PubMed] [Google Scholar]
- 29. Shen C, Wang S, Zhang S, Xu Y, Qian Q, et al. (2013) OsARF16, a transcription factor, is required for auxin and phosphate starvation response in rice (Oryza sativa L.). Plant Cell Environ 36: 607–620. [DOI] [PubMed] [Google Scholar]
- 30. Wang X, Du G, Wang X, Meng Y, Li Y, et al. (2010) The function of LPR1 is controlled by an element in the promoter and is independent of SUMO E3 Ligase SIZ1 in response to low Pi stress in Arabidopsis thaliana. Plant Cell Physiol 51: 380–394. [DOI] [PubMed] [Google Scholar]
- 31. Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bozzo GG, Dunn EL, Plaxton WC (2006) Differential synthesis of phosphate-starvation inducible purple acid phosphatase isozymes in tomato (Lycopersicon esculentum) suspension cells and seedlings. Plant Cell Environ 29: 303–313. [DOI] [PubMed] [Google Scholar]
- 33. Chang L, Ramireddy E, Schmulling T (2013) Lateral root formation and growth of Arabidopsis is redundantly regulated by cytokinin metabolism and signalling genes. J Exp Bot 64: 5021–5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. An L, Zhou Z, Sun L, Yan A, Xi W, et al. (2012) A zinc finger protein gene ZFP5 integrates phytohormone signaling to control root hair development in Arabidopsis. Plant J 72: 474–490. [DOI] [PubMed] [Google Scholar]
- 35. Ito Y, Kurata N (2006) Identification and characterization of cytokinin-signalling gene families in rice. Gene 382: 57–65. [DOI] [PubMed] [Google Scholar]
- 36. Hirose N, Makita N, Kojima M, Kamada-Nobusada T, Sakakibara H (2007) Overexpression of a type-A response regulator alters rice morphology and cytokinin metabolism. Plant Cell Physiol 48: 523–539. [DOI] [PubMed] [Google Scholar]
- 37. Du L, Jiao F, Chu J, Jin G, Chen M, et al. (2007) The two-component signal system in rice (Oryza sativa L.): a genome-wide study of cytokinin signal perception and transduction. Genomics 89: 697–707. [DOI] [PubMed] [Google Scholar]
- 38. Ai P, Sun S, Zhao J, Fan X, Xin W, et al. (2009) Two rice phosphate transporters, OsPht1;2 and OsPht1;6, have different functions and kinetic properties in uptake and translocation. Plant J 57: 798–809. [DOI] [PubMed] [Google Scholar]
- 39. Sun S, Gu M, Cao Y, Huang X, Zhang X, et al. (2012) A constitutive expressed phosphate transporter, OsPht1;1, modulates phosphate uptake and translocation in phosphate-replete rice. Plant Physiol 159: 1571–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang C, Ying S, Huang H, Li K, Wu P, et al. (2009) Involvement of OsSPX1 in phosphate homeostasis in rice. Plant J 57: 895–904. [DOI] [PubMed] [Google Scholar]
- 41. Essigmann B, Guler S, Narang RA, Linke D, Benning C (1998) Phosphate availability affects the thylakoid lipid composition and the expression of SQD1, a gene required for sulfolipid biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci U S A 95: 1950–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Trull MC, Deikman J (1998) An Arabidopsis mutant missing one acid phosphatase isoform. Planta 206: 544–550. [DOI] [PubMed] [Google Scholar]
- 43. Inukai Y, Sakamoto T, Ueguchi-Tanaka M, Shibata Y, Gomi K, et al. (2005) Crown rootless1, which is essential for crown root formation in rice, is a target of an AUXIN RESPONSE FACTOR in auxin signaling. Plant Cell 17: 1387–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Perez-Torres CA, Lopez-Bucio J, Cruz-Ramirez A, Ibarra-Laclette E, Dharmasiri S, et al. (2008) Phosphate availability alters lateral root development in Arabidopsis by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor. Plant Cell 20: 3258–3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang W, Swarup R, Bennett M, Schaller GE, Kieber JJ (2013) Cytokinin induces cell division in the quiescent center of the Arabidopsis root apical meristem. Curr Biol 23: 1979–1989. [DOI] [PubMed] [Google Scholar]
- 46. Robinson WD, Park J, Tran HT, Del Vecchio HA, Ying S, et al. (2012) The secreted purple acid phosphatase isozymes AtPAP12 and AtPAP26 play a pivotal role in extracellular phosphate-scavenging by Arabidopsis thaliana. J Exp Bot 63: 6531–6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tian J, Wang C, Zhang Q, He X, Whelan J, et al. (2012) Overexpression of OsPAP10a, a root-associated acid phosphatase, increased extracellular organic phosphorus utilization in rice. J Integr Plant Biol 54: 631–639. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Physiological results for osarf16/C under 6-BA treatment.
(DOCX)
Physiological evidence for OsARF16 was involved in various cytokinins (kinetin, zeatin) Responses.
(DOCX)
Analysis of shoot/root ratios of P contents in both NIP and osarf16 mutant.
(DOCX)
The expression levels of OsPIN and OsLAX family genes.
(DOCX)
The RT primer sequences 37 cytokinin response genes.
(DOCX)
Primer sequences for phosphorus transporters.
(DOCX)
Primer sequences for the Phosphate Starvation Induced genes ( PSIs ).
(DOCX)
Primer sequences for purple acid phosphatase genes.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.