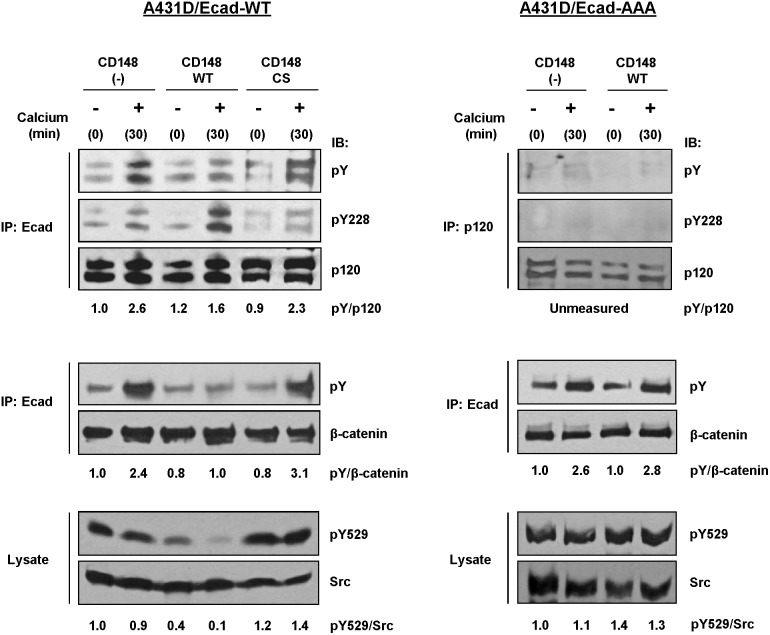
Figure 7. CD148 regulates the tyrosine phosphorylation of p120, β-catenin, and Src upon E-cadherin engagement.
Effects of CD148 in the cadherin adhesion-associated tyrosine phosphorylation of p120, β-catenin, and Src were assessed by a calcium-switch assay and immunoblot analysis using A431D/E-caherin WT (left panels) and A431D/E-cadherin 764AAA (right panels) cells. For p120 and β-catenin, tyrosine phosphorylation of p120 and β-catenin that were co-immunoprecipitated with E-cadherin was assessed by immunoblotting. In A431D/E-cadherin 764 AAA cells, p120 was immunoprecipitated. The membranes were reprobed with p120, β-catenin and Src antibodies and a ratio of phosphorylated to total protein was quantified by densitometry. Data are representative of five independent experiments. CD148 WT, but not CS, reduces the tyrosine phosphorylation of p120, β-catenin, and Src (Y529) upon E-cadherin engagement in A431D/E-cadherin WT cells, while it increases the phosphorylation of Y228 (a Src site) in p120. These effects are not observed in A431D/E-cadherin 764 AAA cells.