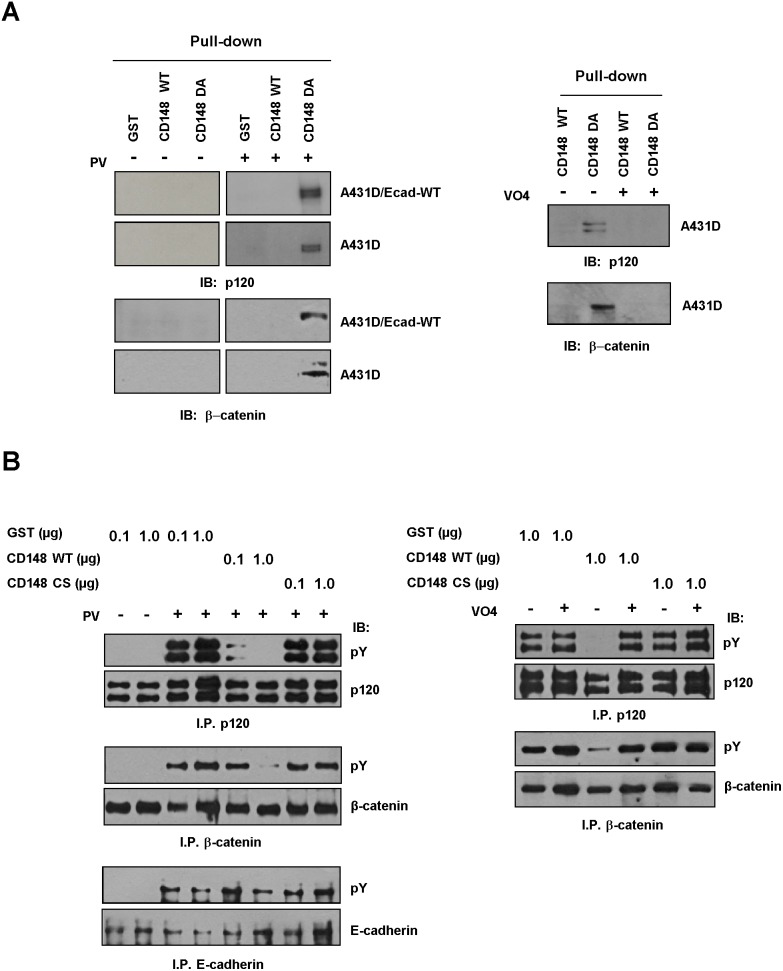
Figure 8. CD148 dephosphorylates p120 and β-catenin in vitro.
A) A431D/E-cadherin WT and A431D cells were treated with (+) or without (−) pervandadate (PV) and cell lysates were incubated with GST or GST-CD148 (WT, DA) proteins. GST-protein complex were pulled-down using glutathione beads and the protein interactions were examined by immunoblotting (left panels). Vandadate (VO4) competition was also assessed (right panels). Substrate-trapping (DA), but not WT, form of CD148 binds to p120 and β-catenin in a phosphorylation dependent manner and these interactions are blocked by vanadate (VO4). B) CD148 dephosphorylation of E-cadherin, p120, and β-catenin was assessed in vitro. E-cadherin, p120, and β-catenin were immunoprecipitated from the pervanadate (PV)-treated or untreated A431D/E-cadherin WT cells. The immunoprecipitates were incubated with GST or GST-CD148 proteins and its effects were assessed by immunoblotting with a pY20 phosphotyrosine antibody (pY) (left panels). The amount of protein was assessed by reprobing the membranes with specific antibodies. Vanadate (VO4) competition was also assessed (right panels). CD148 WT, but not CS, dephosphorylates p120 and β-catenin in a dose dependent manner, while its effects for E-cadherin are limited. CD148 dephosphorylation of p120 and β-catenin is blocked by vanadate (VO4).