Fig. 2. Mechanism of secretion and anchoring of MSCRAMMs to the Gram-positive cell wall.
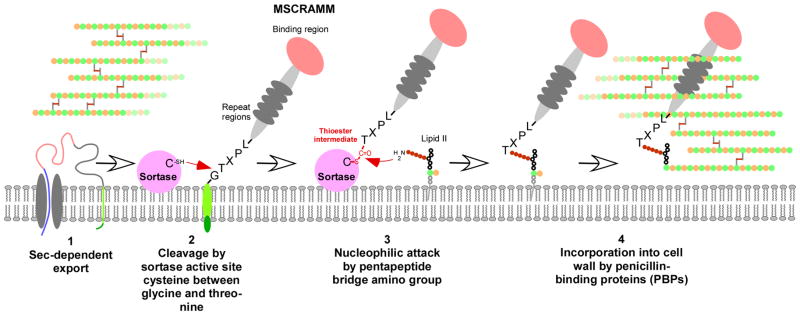
(1) The MSCRAMM protein is secreted by the Sec system, as most other secreted proteins. (However, some MSCRAMMs have dedicated secretion systems, such as S. pyogenes Srr proteins.) This also removes the signal peptide. (2) The active site cysteine thiol of the surface-attached sortase attacks and cleaves between the glycine and threonine residues of the MSCRAMM’s LPXTG motif. (3) The sortase-MSCRAMM thioester-linked intermediate undergoes nucleophilic attack at the thioester carbon by the amino group of an exposed amino acid moiety of the lipid II cell wall biosynythesis precursor (the exposed amino acid of what otherwise would become the peptidoglycan pentapeptide cross bridge in peptidoglycan). (4) The MSCRAMM-lipid II complex is then incorporated by penicillin-binding (i.e., cell wall biosynthesis) proteins into the peptidoglycan network.
