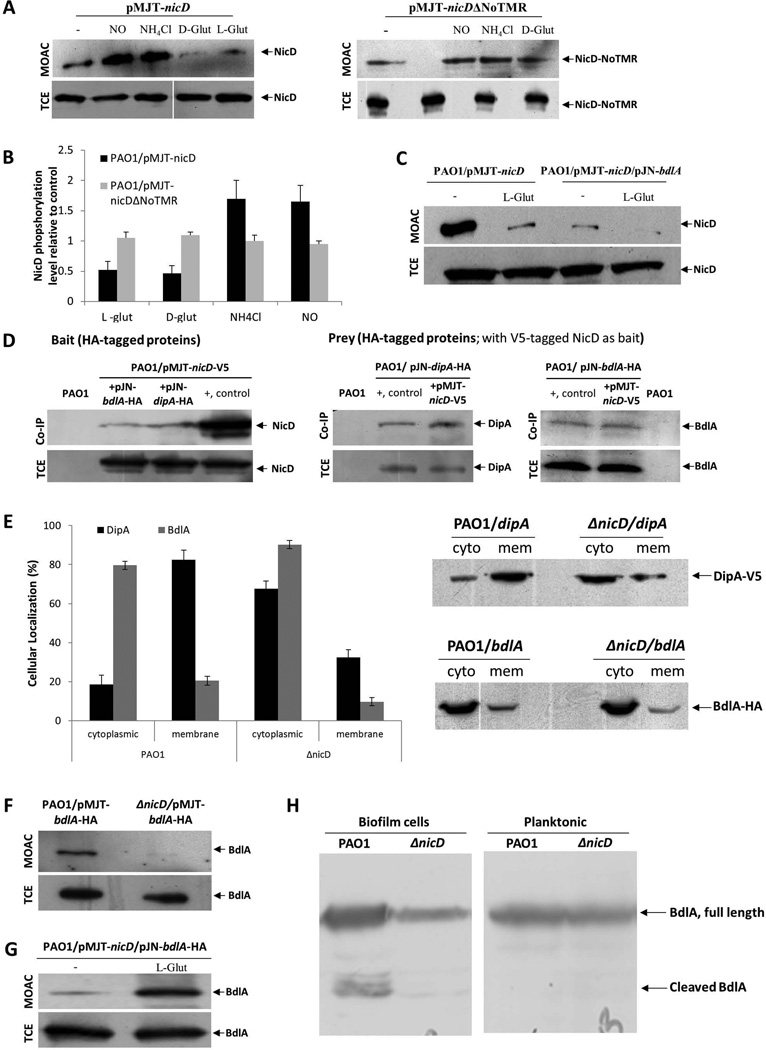
Figure 6. NicD forms a membrane associated complex with DipA and BdlA and contributes to BdlA activation.
(A) Detection of V5-tagged NicD and NicDΔNoTMR in total cell extracts (TCE) or metaloxide affinity chromatography-enriched phosphoproteomes (MOAC) of planktonic P. aeruginosa PAO1/pMJT-nicD and PAO1/pMJT-nicDΔNoTMR cells prior to (−) and following addition of nitric oxide (NO), ammonium chloride (NH4Cl), and D- and L-glutamate (D-glut, L-glut, respectively) by immunoblot analysis using anti-V5 antibodies. A total of 10 µg per TCE was loaded prior to MOAC purification and used as loading control. For MOAC samples, the entire MOAC eluate was loaded. (B) Band intensity was used to determine the relative levels of NicD and NicDΔNoTMR phopshorylation following addition of nitric oxide (NO), ammonium chloride (NH4Cl), and D- and L-glutamate (D-glut, L-glut, respectively) relative to untreated controls (−). (C) Detection of V5-tagged NicD in MOAC-enriched fractions is dependent on the addition of glutamate and the presence of BdlA, as determined by immunoblot analysis using anti-V5 antibodies. NicD phopshorylation levels were determined using of PAO1/pMJT-nicD and PAO1/pMJT-nicD/pJN-bdlA. A total of 10 µg per TCE, obtained prior to MOAC purification, was used as loading control. For MOAC samples, the entire MOAC eluate was loaded. (−) no L-glutamate was added to cells grown planktonically to exponential phase prior to cell lysis. L-glut, L-glutamate was added to cells grown planktonically to exponential phase prior to cell lysis. (D) Immunoblot analysis of in vivo pull-down assays (Co-IP) demonstrating complex formation between NicD, BdlA and DipA using BdlA-HA or DipA-HA either as prey or bait. Total cell extracts obtained from P. aeruginosa PAO1 were used as negative control. Cell extracts containing the protein of interest were used as positive control (+, control). Total cell extracts (TCE) obtained prior to Co-IP were used as loading control. (E) Inactivation of nicD affects the subcellular localization of BdlA and DipA. Detection of subcellular localization was achieved by ultracentrifugation and subsequent analysis by SDS/PAGE and immunoblot analysis using anti-V5 and anti-HA antibodies. A total of 15 µg per cytoplasmic (cyto) and membrane (mem) fraction was loaded. Band intensity was determined using ImageJ to determine the ratio of cellular localization (%) of BdlA and DipA in the absence and presence of NicD. (F) NicD contributes to BdlA phosphorylation. Detection of BdlA-V5 in total cell extracts (TCE) or MOAC-enriched phosphoproteomes (MOAC) of P. aeruginosa PAO1 and ΔnicD biofilms as determined by immunoblot analysis. A total of 10 µg per TCE, obtained prior to MOAC purification, was used as loading control. For MOAC samples, the entire MOAC eluate was loaded. (G) Exposure to L-glutamate enhances BdlA phosphorylation in a NicD-dependent manner. (−) no L-glutamate was added to cells grown planktonically to exponential phase prior to cell lysis. L-glut, L-glutamate was added to cells grown planktonically to exponential phase prior to cell lysis. (H) Non-processive cleavage of BdlA is dependent on the mode of growth and the presence of NicD. Inactivation of nicD impairs BdlA processing under biofilm growth conditions. No BdlA processing is observed under planktonic growth conditions. BdlA was detected by immunoblot analysis. All experiments were carried out at least in triplicate and only representative images are shown. Error bars indicate standard deviation.