Figure 1.
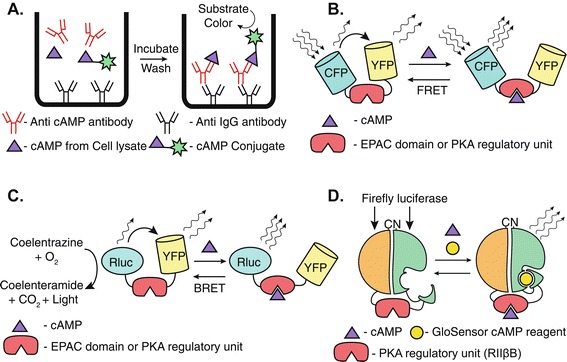
Principles of commonly used cAMP assays. (A) A colorimetric competitive enzyme-linked immunosorbent assay (ELISA) involving incubation of anti-IgG antibody-coated plates with peroxidase labeled cAMP (cAMP-conjugate), anti-cAMP antibody, and cell lysate containing endogenous cAMP. Endogenous cAMP produced upon GPCR stimulation in cells competes with cAMP conjugate for anti-cAMP antibody binding sites. After incubation and washing steps, cAMP-conjugate remaining in the well gives a colorimetric readout upon peroxidase substrate addition. (B) FRET-based sensor design containing cAMP binding domain from either PKA or EPAC protein fused between cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP). In the absence of cAMP, there is FRET between CFP and YFP, whereas cAMP binding to the binding domain leads a conformational change resulting in loss of FRET between CFP and YFP. (C) BRET-based sensor design containing cAMP binding domain from either EPAC or PKA protein fused between Renilla luciferase (Rluc) and YFP. In the absence of cAMP, Rluc utilizes coelenterazine substrate to generate light, part of which is transferred via resonance (BRET) to YFP. Binding of cAMP to the sensor causes a conformational change, thereby abolishing BRET between Rluc and YFP. (D) Design of GloSensor-22F cAMP sensor (adapted from [31]). cAMP-binding domain from PKA regulatory subunit (RIIβB) is fused between N- and C-termini of a permuted firefly luciferase. Binding of cAMP to RIIβB favors a conformational change in two domains of luciferase, which in the presence of its substrate (GloSensor cAMP reagent) gives a luminescent read-out.
