Figure 6.
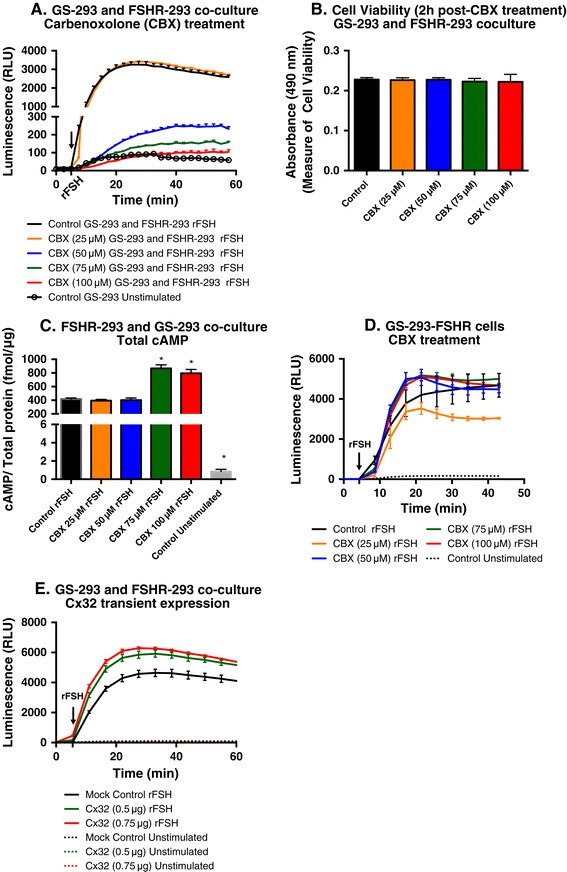
Gap junctions mediate transfer of cAMP from donor to sensor cells. (A) CBX (25, 50, 75 and 100 μM) preincubated co-cultures of GS-293 with FSHR-293 (50,000 cells each) were stimulated with rFSH (200 mIU/ml). Increasing doses of CBX lead to decreasing luminescence, with 100 μM CBX reducing the luminescence to levels similar to unstimulated GS-293 control cells. Data represented as mean of triplicates for one representative experiment ± SEM (positive direction only) with at least three independent repeats. (B) GS-293 and FSHR-293 co-cultures (50,000 cells each) were incubated with CBX (25, 50, 75 and 100 μM) for 2h in assay medium while the control samples had only assay medium without CBX. Cell viability 2h post-CBX treatment shows no statistical difference among samples using one-way ANOVA (p-value =0.9891). (C) Total cAMP concentration in co-cultures of GS-293 and FSHR-293 (150,000 cells each) as assessed by ELISA following stimulation with rFSH (200 mIU/ml) for 20 min. Total cAMP concentration in cells treated with 75 μM and 100 μM CBX was higher (p < 0.0001) than stimulated control while those with 25 μM and 50 μM CBX are similar to the stimulated control. (D) rFSH (200 mIU/ml) stimulation of GS-293-FSHR (50,000 cells) preincubated with higher doses of CBX (50, 75 and 100 μM) moderately increased cAMP production as compared to GS-293-FSHR control cells (preincubated with assay medium without CBX). (E) Co-cultures of GS-293 and FSHR-293 were transfected with increasing amounts of Cx32 plasmid. Co-culture of FSHR-293 and GS-293 transfected with pcDNA3.1 mock plasmid was used as control. Cx32 expression lead to a statistically significant (p-value =0.0017) increase in detection of cAMP by the sensor cells as compared to control stimulated cells. (B, C, D and E) Data represented as mean of triplicates for one representative experiment (± SEM) with at least three independent repeats.
