Figure 1.
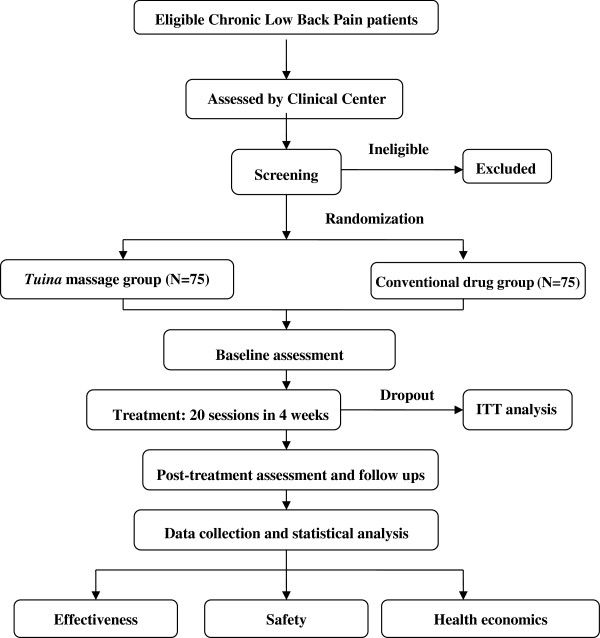
Trial flow chart. The present study is a single center, randomized, conventional drug controlled, open-labeled trial. A total of 150 eligible CLBP patients are anticipated to be included and randomly allocated to either tuina massage treatment group or conventional drug control group, in a 1:1 ratio. Patients in the tuina group receive a four-step massage treatment consisting both structural and relaxation massage. Tuina massage treatment consists of 20 sessions of approximately 20 minutes duration, each administered over a period of four weeks. Patients in the conventional drug control group are instructed to administer ibuprofen. The effectiveness, safety, and health economics of tuina massage versus conventional drugs is analyzed after data collection. The full analysis set including the dropout will be analyzed by the intention-to-treat (ITT) population analysis.
