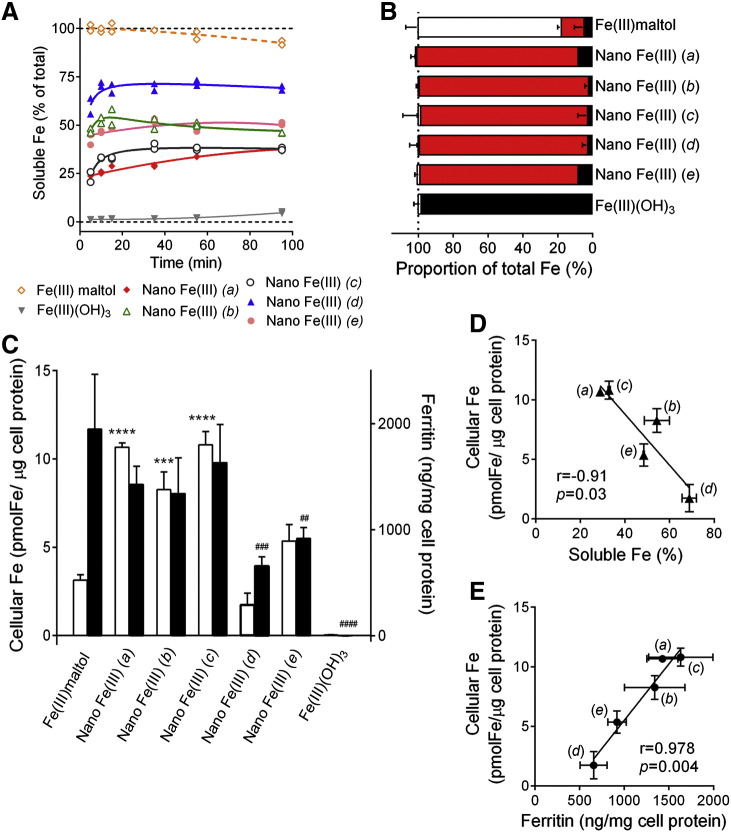
Figure 1.
Solubility and cellular uptake of nano Fe(III). (A) Acid dissolution at pH 3.0 in 9 g/L NaCl. Data are for different formulations of ligand-modified Fe(III) oxo-hydroxides: nano Fe(III) (a)- ligands are tartaric (T) and adipic (A) acids at a ratio 1:1:2 (T:A:Fe) and the material was dried prior to re-suspension; nano Fe(III) (b)—ligands are tartaric (T) and succinic (S) acids at a ratio 1:1:2 (T:S:Fe) and the material was dried prior to re-suspension; nano Fe(III) (c)- ligands are tartaric (T) and succinic (S) acids at a ratio 1:6:2 (T:S:Fe) and the material was dried prior to re-suspension; nano Fe(III) (d)- ligands are gluconic (G) and adipic (A) acids at a ratio 1:1:2 (G:A:Fe) and the material was dried prior to re-suspension; nano Fe(III) (e)- ligands are tartaric (T) and adipic (A) acids at a ratio 1:1:2 (T:A:Fe) and the material was used as a colloidal suspension (i.e. as synthesised) without drying (more details in Table 1). Negative and positive controls are, respectively, unmodified Fe(III) oxo-hydroxide (Fe(III)(OH)3) and Fe(III) maltolate (Fe(III) maltol). Data are shown for the two independent replicates. Dotted black lines show 0 and 100% solubility. All data were obtained by measuring the iron concentration in the supernatant following ultrafiltration (Mr 3000 cut-off). (B) Dispersion of the different iron materials in the BSS uptake medium, used for the Caco-2 cell experiments, as assessed by the fractional percentage of microparticulate (black), nanoparticulate (red) and soluble (white) Fe for each Fe material. Values are mean ± SD of three independent replicates. (C) Cellular iron (open bars) and ferritin (closed bars) levels in Caco-2 cells 23 hours following a one hour exposure to 0.5 mM Fe as unmodified Fe(III) oxo-hydroxide (Fe(III)(OH)3), ligand-modified Fe(III) oxo-hydroxides (nano Fe(III) (a-e)), or soluble Fe(III) maltolate (Fe(III)maltol). Results are mean ± SD of three independent experiments (each condition tested in triplicate wells within each experiment). Statistical comparisons in relation to the soluble control, Fe(III) maltol: ***, P = 0.0008; ****, P < 0.0001 for cellular iron; ##, P = 0.003; ###, P = 0.0002 and ####, P < 0.0001 for ferritin. (D) Pearson's correlation between the solubility of nano Fe(III) at pH3.0 after 15 minutes and cellular iron levels of Caco-2 cells following exposure to nano Fe(III). (E) Pearson's correlation between cellular ferritin levels and cellular iron levels in Caco-2 cell monolayers following exposure to nano Fe(III). For panels (D) and (E), values are mean ± SD, in both the X and Y directions. Where not apparent, the error bars are smaller than the symbol size. Data points are labelled with the nano Fe(III) preparation codes (a–e).