Figure 3.
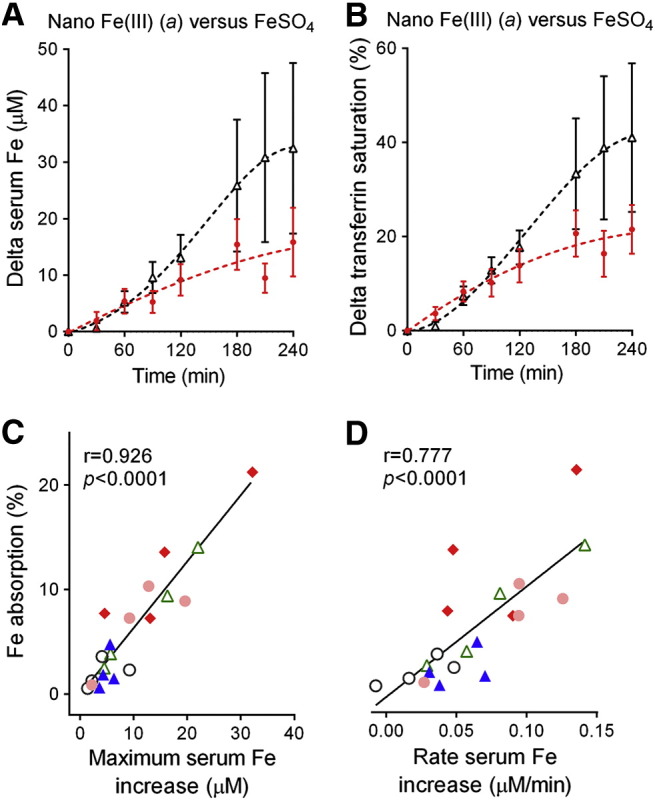
Serum iron absorption following ingestion of a single-dose of the different Fe materials in iron-deficient women. Serum iron increase (A) and transferrin saturation increase (B) following a single dose of nano Fe(III) preparation (a) (closed circles) and Fe(II) sulfate (open triangles). Values are shown as mean and error bars represent SEM (n = 4). Transferrin saturation was defined as serum iron divided by total iron binding capacity and expressed as a percentage. (C-D) Pearson's correlation between percentage of iron absorption (calculated from the red cell incorporation of 58Fe) and maximum serum Fe increase (C) or rate of serum iron increase (D) for the five nano Fe(III) materials. Data points correspond to each individual study participant and are colour coded to reflect the different nano Fe(III) preparations: closed diamonds, nano Fe(III) (a); open triangles, nano Fe(III) (b); open circles, nano Fe(III) (c); closed triangles, nano Fe(III) (d); closed circles, nano Fe(III) (e). Nano Fe(III) (a)—ligands are tartaric (T) and adipic (A) acids at a ratio 1:1:2 (T:A:Fe) and the material was dried prior to re-suspension; nano Fe(III) (b)—ligands are tartaric (T) and succinic (S) acids at a ratio 1:1:2 (T:S:Fe) and the material was dried prior to re-suspension; nano Fe(III) (c)—ligands are tartaric (T) and succinic (S) acids at a ratio 1:6:2 (T:S:Fe) and the material was dried prior to re-suspension; nano Fe(III) (d)—ligands are gluconic (G) and adipic (A) acids at a ratio 1:1:2 (G:A:Fe) and the material was dried prior to re-suspension; nano Fe(III) (e)— ligands are tartaric (T) and adipic (A) acids at a ratio 1:1:2 (T:A:Fe) and the material was used as a colloidal suspension (i.e. as synthesised) without drying (more details in Table 1).
