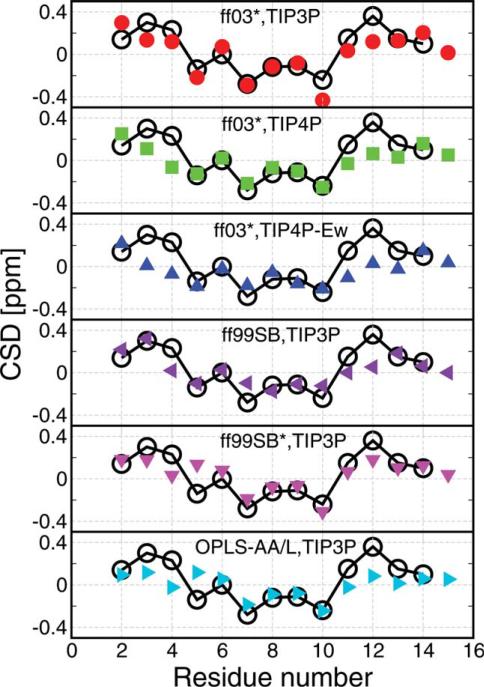
Figure 6.
Comparison with experimental NMR chemical shift deviations (CSD).
The calculated Hα CSD (obtained experimentally by subtracting the random coil shift) is shown for 278 K replicas by using CamShift algorithm.60 Black empty circles are the experimental data at 278 K.49 α-Helical structure is usually associated with a negative CSD and extended structures with a positive CSD.63 Note that although two α proton shifts can be measured for Gly, CamShift reports only a single value. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]