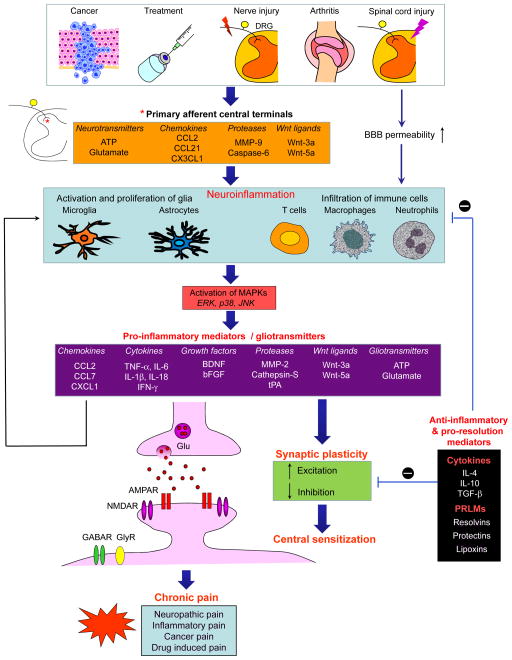
Figure 2. Neuroinflammation in the spinal cord drives chronic pain via neuron–glial interactions and central sensitization.
Chronic pain, such as nerve injury and spinal cord injury-induced neuropathic pain, arthritis-induced inflammatory pain, cancer pain, and drug treatment-induced pain is a result of neuroinflammation in the spinal cord. This neuroinflammation is triggered by activity-dependent release of glial activators (that is, neurotransmitters, chemokines and proteases, and wnt ligands) from the central terminals of primary afferent neurons and/or by disruption of BBB. Neuroinflammation is characterized by the activation of microglia and astrocytes, the infiltration of immune cells to the PNS (e.g., DRG) and CNS (e.g., spinal cord), and the production of inflammatory and glial mediators such as proinflammatory cytokines and chemokines, growth factors and gliotransmitters (glutamate and ATP). These glial mediators can powerfully modulate excitatory and inhibitory synaptic transmission, leading to central sensitization and enhanced chronic pain states. Glial mediators can further act on glial and immune cells to facilitate neuroinflammation via autocrine and paracrine routes. Furthermore, neuroinflammation also generates anti-inflammatory cytokines and pro-resolution lipid mediators (PRLMs) to normalize neuroinflammation, synaptic plasticity and abnormal chronic pain.