Abstract
BACKGROUND & AIMS
The hepatocyte-derived hormone fibroblast growth factor 21 (FGF21) is a hormone-like regulator of metabolism. The NAD+-dependent deacetylase SIRT1 regulates fatty acid metabolism through multiple nutrient sensors. Hepatic overexpression of SIRT1 reduces steatosis and glucose intolerance in obese mice. We investigated mechanisms by which SIRT controls hepatic steatosis in mice.
METHODS
Mice with liver-specific disruption of Sirt1 (SIRT1 LKO mice) and their wild-type littermates (controls) were divided into groups that were placed on normal chow diets, fasted for 24 hrs, or fasted for 24 hrs and then fed for 6 hrs. Liver tissues were collected and analyzed by histologic, gene expression profile, and real-time PCR assays. Human HepG2 cells were incubated with pharmacologic activators of SIRT1 (resveratrol or SRT1720) and assessed by mitochondrial oxidation and immunoblot analyses. FGF21 was overexpressed in SIRT1 LKO mice using an adenoviral vector. Energy expenditure was assessed by indirect calorimetry.
RESULTS
Fasting induced lipid deposition in livers of control mice, but severe hepatic steatosis in SIRT1 LKO mice. Gene expression analysis showed that fasting upregulated FGF21 in livers of control, but not SIRT1 LKO mice. Decreased hepatic and circulating levels of FGF21 in fasted SIRT1 LKO mice were associated with reduced hepatic expression of genes involved in fatty acid oxidation and ketogenesis, and increased expression of genes that control lipogenesis, compared with fasted control mice. Resveratrol or SRT1720 each increased transcriptional activity of the FGF21 promoter (–2070/+117) and levels of FGF21 mRNA and protein in HepG2 cells. Surprisingly, SIRT1 LKO mice developed late-onset obesity with impaired whole-body energy expenditure. Hepatic overexpression of FGF21 in SIRT1 LKO mice increased expression of genes that regulate fatty acid oxidation, decreased fasting-induced steatosis, reduced obesity, increased energy expenditure, and promoted browning of white adipose tissue.
CONCLUSION
SIRT1-mediated activation of FGF21 prevents liver steatosis caused by fasting. This hepatocyte-derived endocrine signaling appears to regulate expression of genes that control a brown fat-like program in white adipose tissue, energy expenditure, and adiposity. Strategies to activate SIRT1 or FGF21 might be used to treat fatty liver disease and obesity.
Keywords: liver-specific disruption of Sirt1, hepatocyte-derived hormone, metabolic homeostasis, obesity
Introduction
Deregulation of metabolic homeostasis is a common characteristic of metabolic disorders such as fatty liver disease, obesity, and diabetes. The liver functions as a major metabolic buffering system for metabolic homeostasis, allowing extra-hepatic tissues such as the brain and heart to function normally under nutrient stress and deprivation1. An important element in the transcriptional response to nutrient deprivation is the NAD+-dependent deacetylase SIRT1 that tightly regulates fatty acid metabolism through multiple nutrient sensors such as AMPK, SREBP-1, and PGC-1α2-4. Polyphenolic SIRT1 activators, including resveratrol and the synthetic polyphenol S17834, prevent hepatic steatosis and hyperlipidemia in mice with type 1 and type 2 diabetes5, 6. Hepatic overexpression of SIRT1 ameliorates hepatic steatosis and glucose intolerance in obese mice7. However, the underlying mechanism of hepatic SIRT1 actions remains incompletely understood. In our effort to identify a novel downstream regulator of SIRT1, gene expression chip assays revealed fibroblast growth factor 21 (FGF21), the hepatocyte-derived hormone, as the most markedly downregulated gene in liver-specific SIRT1 knockout (SIRT1 LKO) mice.
FGF21, a fasting-induced hepatokine, is rapidly gaining interest as a metabolic regulator8, 9, although the mechanism of nutrient regulation of FGF21 is unclear. FGF21 is expressed predominantly in the liver, adipose tissue, and pancreas, with most circulating FGF21 originating from liver10. The autocrine/paracrine and endocrine actions of FGF21 hormone are mediated through FGF receptors complexed with β-klotho11. Both pharmacological administration of FGF21 and transgenic overexpression of FGF21 in mice protect against body weight gain and metabolic dysfunction in diabetic rodents, monkeys, and humans8, 12-16. Previous studies reported that SIRT1 regulates FGF21 expression in hepatocytes in vitro and in vivo17,18. However, the relative contribution of FGF21 to SIRT1's effects on overall energy metabolism has not been investigated. The present study characterizes FGF21 as a critical downstream regulator of SIRT1 that protects against hepatic steatosis, enhances expression of brown fat-like genes in white adipose tissue, and increases whole-body energy expenditure. Our in vivo and in vitro studies illustrate that (1) hepatic SIRT1 is required for fasting-induced production and secretion of FGF21 in the liver; (2) defective FGF21 caused by hepatic SIRT1 ablation exacerbates fasting-induced hepatic steatosis by impairing fatty acid oxidation and increasing lipogenesis; (3) FGF21 is essential for SIRT1 to stimulate hepatocyte fatty acid oxidation; (4) hepatic overexpression of FGF21 enhances systemic energy expenditure and ameliorates obesity.
Materials and Methods
Animals
Hepatocyte-specific deletion of the SIRT1 gene in mice (SIRT1 LKO) was achieved by crossing albumin-Cre recombinase transgenic mice with floxed SIRT1 ex4 mice containing the deleted SIRT1 exon 4, which encodes 51 amino acids of the conserved SIRT1 catalytic domain, as described previously19. The protocol for this study was approved by the Boston University Medical Center Institutional Animal Care and Use Committee.
Animal fasting and refeeding experiments
SIRT1 LKO mice and their WT littermates were divided into three groups: fed, fasted, and refed. The fed group was placed on a normal chow diet; the fasted group was fasted for 24 h; and the refed group was fasted for 24 h and then fed for 6 h.
In vivo adenoviral gene transfer
The adenovirus producing full-length FGF21 was generated and purified as described previously6, 20. Adenovirus-mediated overexpression of FGF21 in vivo was accomplished via tail vein injection as described previously2, 7, 20.
Statistical analysis
Values are expressed as the mean ± S.E.M. Statistical significance was evaluated using an unpaired two-tailed t-test or a one-way ANOVA for greater than two groups. Differences were considered significant at the P<0.05 level.
Results
Hepatocyte-specific deletion of SIRT1 in mice increases the susceptibility to fasting-induced fatty liver
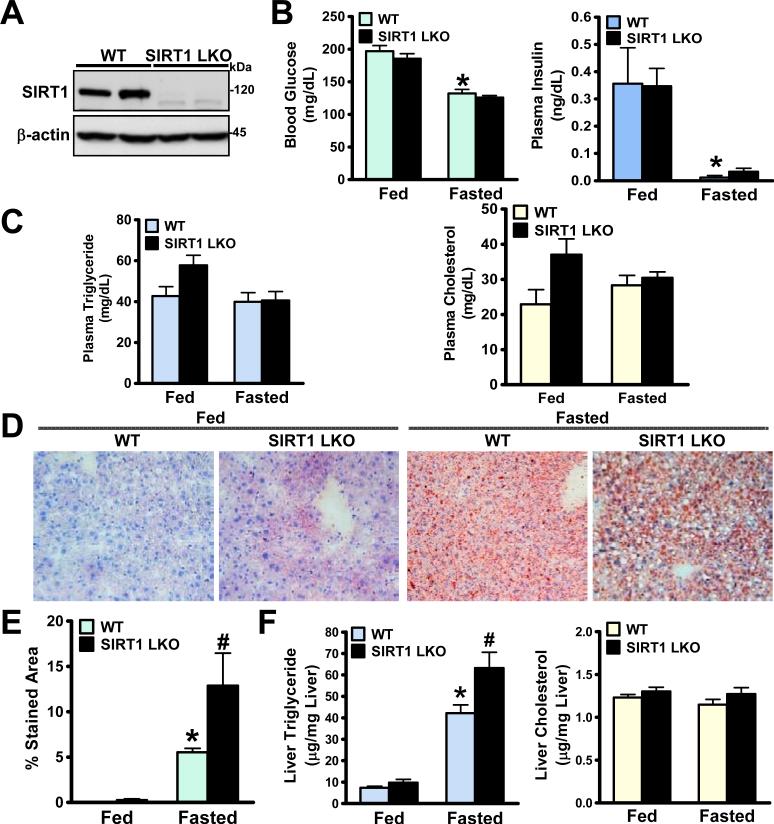
To explore whether liver-tissue specific SIRT1 is a driving force to maintain lipid homeostasis, metabolic phenotypes of hepatic SIRT1 LKO mice were characterized. As shown in Fig. 1, blood glucose and plasma insulin levels were comparable between WT and SIRT1 LKO mice in both fed and fasted states. In the fed state, plasma cholesterol and triglyceride levels were slightly higher in SIRT1 LKO mice compared to those in the WT littermates. In the fasted state, plasma lipids were similar between WT and SIRT1 LKO mice. In liver, fasting induced lipid deposition in WT mice, as shown by Oil Red O staining and direct triglyceride measurements, Strikingly, pronounced hepatic steatosis was observed in fasted SIRT1 LKO mice, as evidenced by increased stained areas and elevated hepatic triglyceride content. Hepatic cholesterol content was comparable amongst groups. These data indicate that hepatic SIRT1 may play a critical role in regulating lipid metabolism under fasting conditions.
Fig. 1. Hepatocyte-specific deletion of SIRT1 in mice increases susceptibility to developing fasting-induced fatty liver.
A. Representative immunoblots for SIRT1 in two mouse livers from each group are shown. The expected deletion mutant protein in SIRT1 LKO mice, which migrates slightly faster than WT SIRT1, was visualized by immunoblotting. B. Blood glucose and plasma insulin levels in WT and SIRT1 LKO mice at 6- and 7-months of age that were fed a normal diet (Fed) or fasted for 24 h (Fasted) (n = 10-18). C. Plasma triglyceride and cholesterol levels in mice (n = 4-6). D and E. Hepatic steatosis was assessed by Oil Red O staining and quantified by measuring Oil Red O stained areas. F. Hepatic triglyceride and cholesterol levels in WT and SIRT1 LKO mice (n=4-6). Results are presented as the mean ± S.E.M. *P<0.05, vs. the fed WT mice; #P<0.05, vs. the fasted WT mice.
Loss of SIRT1 in the liver suppresses fasting-induced gene expression, production, and secretion of hepatic FGF21
As part of a directed screen to identify a novel secreted protein that could be modulated by genetic manipulation of hepatic SIRT1, gene expression chip analysis showed that FGF21 was reduced by 83% in livers of SIRT1 LKO mice compared to those of WT mice under feeding conditions. Fasting resulted in an 11.7-fold induction of FGF21 in livers of WT mice. Hepatic SIRT1 deletion ablated approximately 60% of the FGF21 induction under fasting conditions (Table S2). PPARα gene expression was increased ~2-fold in WT mice in response to fasting and decreased 40% in SIRT1 LKO mice. However, gene expression profiles of pro-inflammatory mediators, cytokines, growth factors, oxidative stress, and redox modulators were comparable between both genotypes of mice under nutrient stress conditions (Table S2).
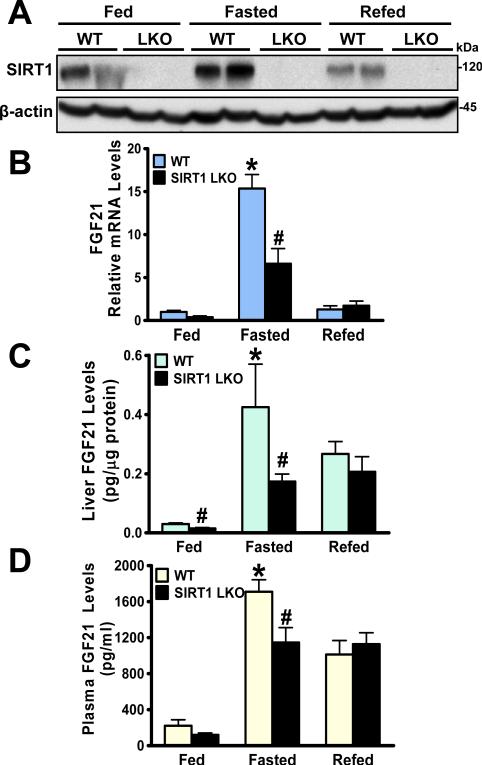
We next determined whether hepatic SIRT1 deficiency might interfere with fasting-induced production and release of hepatic FGF21. As shown in Fig. 2, protein expression of hepatic SIRT1 was induced by nutrient deprivation and repressed by nutrient availability. Similar to the gene chip data (Table 2), mRNA levels of FGF21 were robustly increased 15-fold upon fasting and decreased nearly 50% upon refeeding as observed previously9. Consistently, FGF21 protein production was increased 5-fold in WT mice following a 24 h fast, and this induction was decreased ~40% in SIRT1 LKO mice. These results suggest that hepatic SIRT1 and FGF21 both are physiologically regulated by a feeding-fasting-refeeding cycle, and that SIRT1 is a major regulator of fasting-inducible FGF21. Importantly, plasma FGF21 levels were similarly increased 8-fold in WT mice in response to fasting, and this induction was suppressed ~40% in SIRT1 LKO mice. Additionally, a moderate but consistent reduction of FGF21 production and secretion was also seen in the fed SIRT1 LKO mice. The effect of refeeding on FGF21 was comparable between both genotypes. Therefore, hepatic SIRT1 is necessary for prolonged fasting-induced gene transcription, protein production, and secretion of FGF21.
Fig. 2. Production and secretion of hepatic FGF21 are reduced in SIRT1 LKO mice in response to nutrient deprivation.
SIRT1 LKO mice and their WT littermates at 6- and 7-months of age (n = 4-7) were subjected to feeding (Fed), fasting for 24 h (Fasted), or refeeding for 6 h following a 24-h fast (Refed). A. Hepatic SIRT1 expression is increased by fasting and decreased by refeeding in WT mice, and the induction is eliminated in SIRT1 LKO mice (n = 4-7). B and C. Fasting-induced gene expression and protein production of hepatic FGF21 are diminished in SIRT1 LKO mice (n = 4-7), as determined by real-time PCR and ELISA assays. D. The circulating levels of FGF21 are robustly elevated by fasting in WT mice, and the induction is impaired in SIRT1 LKO mice. *P<0.05, vs. the fed WT mice; #P<0.05, vs. the fasted WT mice.
Loss of SIRT1 in the liver alters expression of key genes involved in lipid metabolism
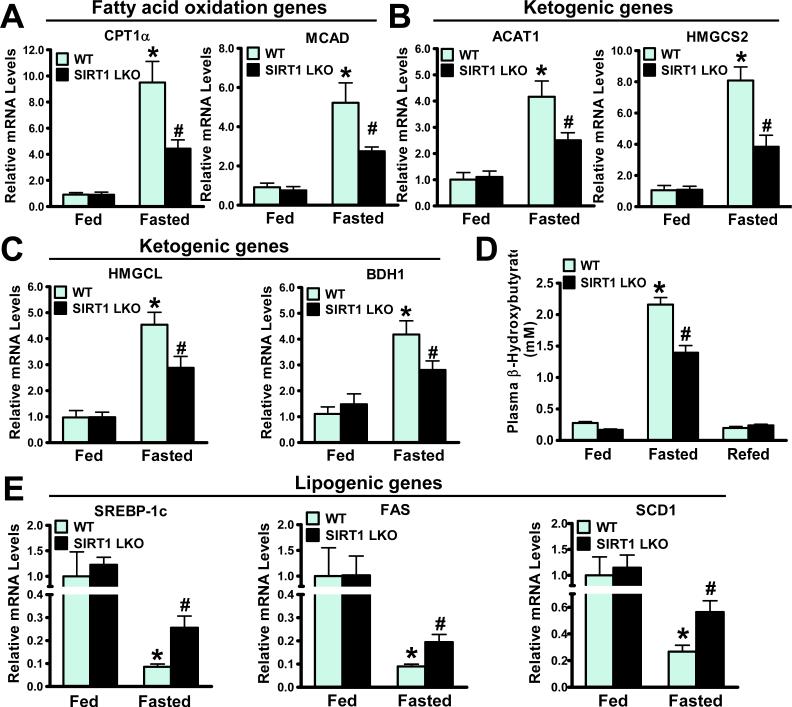
It has been shown that FGF21 regulates hepatic fatty acid oxidation and ketogenesis9, 21. To determine the functional consequence of defective FGF21 in SIRT1 LKO mice, we measured the expression of key genes involved in lipid metabolism. mRNA amounts of rate-limiting enzymes of fatty acid oxidation, carnitine palmitoyl transferase 1α (CPT1α) and medium-chain acyl-CoA dehydrogenase (MCAD), were increased approximately 9- and 5-fold in fasted WT livers respectively, but upregulated only 4- and 2.5-fold in fasted SIRT1 LKO livers respectively (Fig. 3A); these changes were previously demonstrated in hepatic FGF21 knockdown mice fed a ketogenic diet9. The results suggest that induction of hepatic FGF21 and β-oxidation during fasting is largely diminished in SIRT1 LKO mice, a metabolic defect that could explain the pronounced hepatic steatosis found in SIRT1 LKO mice.
Fig 3. Expression of metabolic genes involving fatty acid oxidation, ketogenesis, and lipogenesis is altered in livers of SIRT1 LKO mice.
A. Hepatic expression of key genes of fatty acid oxidation such as CPT1α and MCAD is induced by fasting in WT mice and inhibited in SIRT1 LKO mice (n = 4-7). B and C. Fasting induced expression of ketogenic enzymes including ACAT1, HMGCS2, HMGCL, and BDH1 is suppressed in livers of SIRT1 LKO mice. D. Effects of fasting and refeeding on circulating ketone bodies in mice (n = 5-10). E. mRNA amounts of SREBP-1c and key lipogenic enzymes including FAS and SCD1 are upregulated in livers of SIRT1 LKO mice. n = 4-7, *P<0.05, vs. the fed WT mice; #P<0.05, vs. the fasted WT mice.
Consistent with the concomitant induction of hepatic SIRT1 and FGF21 by nutrient deprivation (Fig. 2), mRNA levels of several enzymes required for ketone body synthesis, including acetyl-CoA acetyltransferase 1 (ACAT1), 3-hydroxy-3-methylglytary-CoA synthase 2 (HMGCS2), HMG-CoA lyase (HMGCL), and β-hydroxybutyrate dehydrogenase 1 (BDH1), were increased by 4- to 8-fold in WT mice under fasting conditions. Consequently, plasma β-hydroxybutyrate levels were increased 8-fold by fasting and fully suppressed by refeeding. Strikingly, fasting-induced adaptive ketogenesis was markedly blunted in SIRT1 LKO mice (Fig. 3B-D). Collectively, hepatic SIRT1 deficiency inhibits hepatic fatty acid utilization and energy supply to extra-hepatic tissues, likely via suppression of FGF21-regulated processes of β-oxidation and ketogenesis.
Our previous studies demonstrated that SIRT1 suppresses high glucose-induced fatty acid synthase (FAS) expression and lipid accumulation in HepG2 cells2. To further delineate the mechanisms responsible for the steatotic phenotype in SIRT1 LKO mice, gene expression of a key lipogenic transcription factor, the sterol regulatory element binding protein-1c (SREBP-1c), and its target genes, was assessed by real-time PCR. mRNAs encoding SREBP-1c, FAS, and stearoyl-CoA desaturase (SCD1) were upregulated over 2-fold in fasted SIRT1 LKO mice (Fig. 3E). Taken together, hepatic steatosis in SIRT1 LKO mice with triglyceride accumulation is likely attributed to reduction of fatty acid oxidation and stimulation of fatty acid synthesis.
FGF21 is essential for SIRT1 to regulate hepatocyte fatty acid oxidation
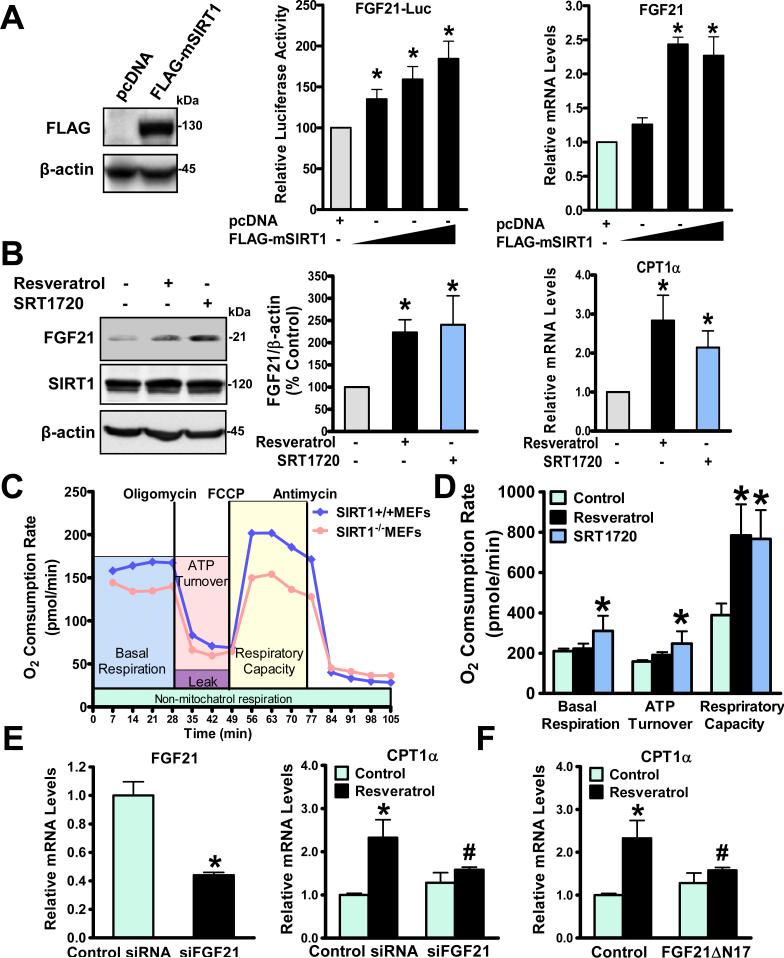
To investigate a causal relationship between SIRT1 and FGF21 in a cell autonomous manner, human HepG2 cells were used to investigate the transcriptional regulation of FGF21 by SIRT1 because this cell line expresses both genes2, 20 and responds well to SIRT1 activators to lower hepatocellular lipids2. As shown in Fig. 4A-D, luciferase-based reporter assays with a human FGF21 promoter (-2090 to +117)20 showed that FGF21 promoter-driven reporter activity was progressively stimulated by increasing expression of SIRT1. FGF21 mRNA levels were dose-dependently elevated by SIRT1 overexpression in HepG2 cells, consistent with the previous observation in primary hepatoctyes17. To determine the effect of SIRT1-induced FGF21 on fatty acid oxidation, immunoblotting analysis showed that protein production of FGF21 was induced over 2-fold by SIRT1 activators including resveratrol (10 M) and SRT1720 (5 M), an effect which was well correlated with their ability to elevate gene expression of CPT1[.alpha]. In parallel, cellular oxygen consumption rates (OCR), an index of mitochondrial oxidation function and a readout of energy expenditure in vitro 22, were measured using an extracellular flux analyzer of live cells in real time. Compared to those of SIRT1+/+ MEFs, the bioenergetic profiles of SIRT1-/-MEFs revealed a decrease in basal and maximal mitochondrial respiratory capacity, consistent with repressed CPT1α and MCAD expression found in SIRT1 LKO livers (Fig. 3A) and decreased rates of fatty acid oxidation seen in SIRT1−/− MEFs3. Conversely, basal respiration was moderately but significantly increased by SRT1720, and maximal respiration caused by uncoupling agent FCCP was further stimulated over 2-fold by SRT1720 (5 M) in HepG2 cells, an effect also seen with resveratrol (10 M). Notably, induction of CPT1α by SIRT1 activators was closely correlated with their ability to elevate oxygen consumption rates. Taken together, our data reveal that SIRT1 potently induces FGF21 and fatty acid oxidation in vitro.
Fig. 4. SIRT1 stimulates mitochondrial fatty acid oxidation in an FGF21-dependent manner in HepG2 cells.
A. Increased expression of SIRT1 progressively stimulates transcriptional activity of the FGF21 (-2090/+117) promoter-driven reporter and elevates FGF21 gene expression. Overexpression of FLAG-tagged mouse SIRT1 (FLAG-mSIRT1) in HEK293T cells is confirmed by immunoblots. B. Protein production of FGF21 and mRNA amounts of CPT1α are stimulated by SIRT1 activators resveratrol (10 μM) and SRT1720 (5 μM). C. Oxygen consumption rates (OCRs) are determined by analyzing a bioenergetics profile of SIRT1+/+ and SIRT1-/- MEFs using the Seahorse Bioscience. The OCRs of basal respiration, ATP turnover, and respiratory capacity were measured and calculated as averages for each phase. D. Mitochondrial OCR is increased by SIRT1 activators. E. The ability of resveratrol (10 μM) to induce CPT1α is abrogated by siRNA-mediated FGF21 knockdown in HepG2 cells. F. Resveratrol-induced CPT1α expression is diminished by a competitive inhibitor of FGF21 (FGF21∆N17, 100nM) in HepG2 cells. n = 3-4, *P < 0.05, vs control cells; #P<0.05, vs treatment cells.
To investigate whether the autocrine/paracrine effect of FGF21 is responsible for the upregulation of fatty acid oxidation by SIRT1, we found that expression of CPT1α was increased ~2-fold by resveratrol, but such an induction was largely diminished by siRNA-mediated knockdown of FGF21 (Fig. 4E). By using a competitive antagonist of FGF21, an N-terminally truncated FGF21 protein (ΔN17) that was previously described to block in vivo FGF21 signaling14, we observed that the stimulatory effect of resveratrol on CPT1α was significantly blocked by the FGF21ΔN17 mutant (Fig. 4F). These studies of the genetic and pharmacological manipulation of SIRT1 or FGF21 define FGF21 as a novel downstream mediator of SIRT1 to stimulate hepatocyte β-oxidation.
Hepatic overexpression of FGF21 ameliorates hepatic steatosis in SIRT1 LKO mice
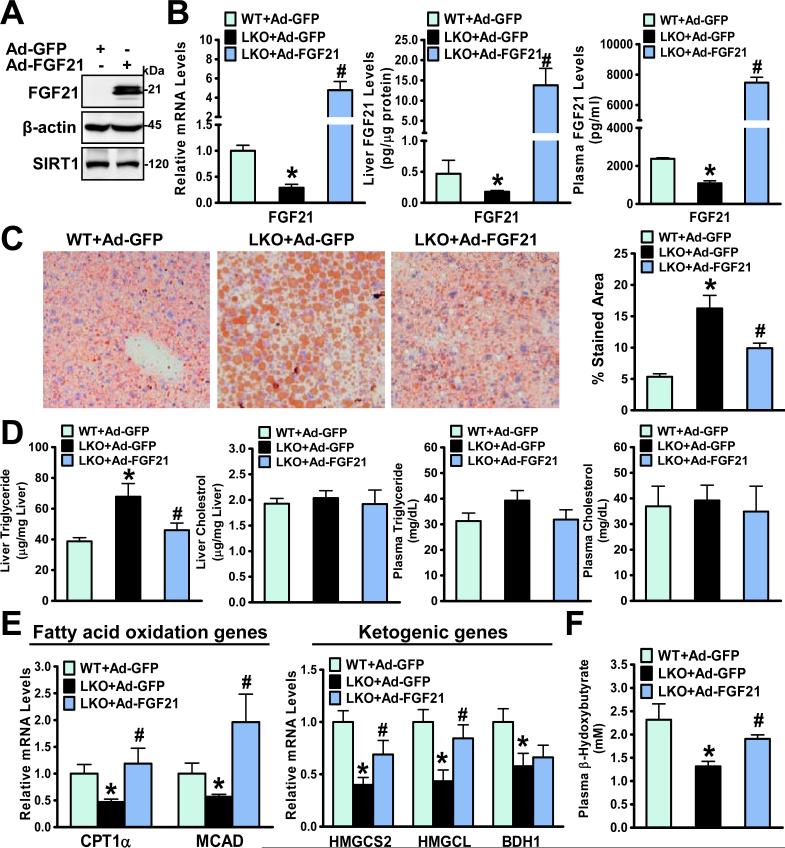
Given that fasting-induced fatty liver is associated with FGF21 insufficiency in SIRT1 LKO mice (Fig. 2), rescue experiments with Ad-FGF21 were performed to determine the effect of FGF21 gain-of-function on the steatotic phenotype of SIRT1 LKO mice. As shown in Fig. 5, in vivo adenoviral gene transfer of FGF21 into SIRT1 LKO mice was successfully accomplished and evidenced by robustly elevated hepatic production and circulating levels of FGF21 in mice at two weeks post-injection. Fasting-induced fatty liver in SIRT1 LKO mice was attenuated by FGF21 overexpression, as reflected by reductions in Oil Red O-stained areas and hepatic triglyceride content. Plasma triglyceride and cholesterol levels were not altered (Fig. 5D). Remarkably, reduced fatty acid oxidation genes (CPT1α and MCAD) in the fasted SIRT1 LKO mice were normalized by reconstitution of FGF21, and the impairment of the transcription of ketogenic genes (HMGCS2 and HMGCL) in SIRT1 LKO mice was also restored. Consequently, the reduction of plasma β–hydroxybutyrate concentrations in fasted SIRT1 LKO mice was counteracted by FGF21 overexpression, reaching to nearly normal levels in control GFP-expressing mice. Ad-FGF21 treatment did not alter SREBP1c and FAS expression in livers of SIRT1 LKO mice (Fig. S1). Therefore, it appears that hepatic SIRT1 deficiency exacerbates fasting-induced hepatic steatosis largely through hepatic impairment of FGF21 and fatty acid oxidation.
Fig. 5. Hepatic overexpression of FGF21 rescues hepatic steatosis and metabolic defects in fasted SIRT1 LKO mice.
A. An adenovirus vector encoding FGF21 (Ad-FGF21) is overexpressed in HEK293A cells. B. Adenoviral overexpression of FGF21 in SIRT1 LKO mice (n = 4-7) is evidenced by increased hepatic gene expression, protein production, and circulating levels of FGF21. C and D. Fasting-induced hepatic steatosis in SIRT1 LKO mice is ameliorated by hepatic overexpression of FGF21, as indicated by decreased Oil Red O stained areas and lowered hepatic triglyceride levels. E and F. Suppression of hepatic fatty acid oxidation and ketogenesis in SIRT1 LKO mice is prevented by hepatic overexpression of FGF21. *P<0.05, vs Ad-GFP-injected WT mice; #P<0.05, vs Ad-GFP-injected SIRT1 LKO mice.
SIRT1 LKO mice develop late-onset obesity with decreased whole-body energy expenditure
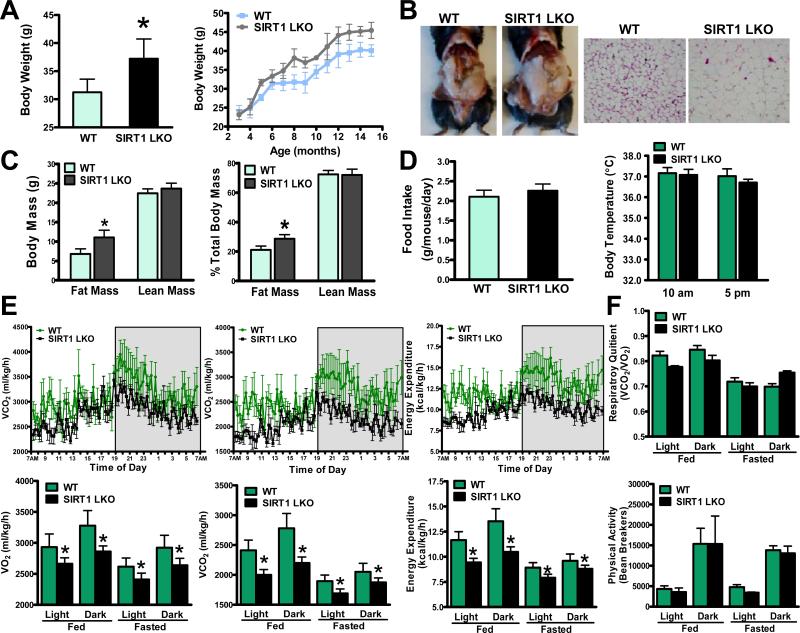
FGF21 has emerged as an important regulator of energy expenditure and body weight12. Since circulating FGF21 was reduced in SIRT1 LKO mice (Fig. 4A), we sought to determine whether hepatocyte-specific deletion of SIRT1 would affect energy expenditure and adiposity. As shown in Fig. 6A-C, compared with WT littermates fed a standard chow diet, SIRT1 LKO mice were more susceptible to late-onset body weight gain, as evidenced by a moderate but statistically significant increase in body weight. SIRT1 LKO mice appeared to have increased adiposity with more visible fat, possibly due to increased adipocyte cell size. Body composition analysis showed that SIRT1 LKO mice had increased fat mass without changed lean mass.
Fig. 6. SIRT1 LKO mice are susceptible to developing obesity with increased adiposity and decreased total oxygen consumption and energy expenditure.
A. Chow-fed SIRT1 LKO mice at 6- and 7-months of age (n = 8) gain more body weight. Body weight curves of WT and SIRT1 LKO mice over a 15-month period (n = 8–16). B. A representative photograph of increased adiposity and adipocyte size in white adipose tissue (WAT) of SIRT1 LKO mice. C. Body composition analysis of WT and SIRT1 LKO mice. D. Food intake (n = 10-12) and body temperature (n = 6) are similar between WT and SIRT1 LKO mice. E. The rates of VO2, VCO2, and energy expenditure are measured by comprehensive metabolic monitoring in mice (n = 4) over a 24-h period with food and over a 24-h fast and normalized to lean body mass. The upper panels represent circadian changes in metabolic parameters in mice in the fed state during light and dark cycles, as indicated by the white and gray areas. The lower panels represent metabolic rates in mice in fed and fasted states. F. Respiratory quotient (RQ) and locomotor activity are determined in metabolic cages. The physical activity is expressed as total counts measured by summing X and Y beam breaks, and the bar graph represents average locomotor activity over a 12-h block of light and dark phases. *P<0.05, vs. WT mice.
To understand the physiological mechanisms by which SIRT1 LKO mice are more susceptible to developing obesity, the major components of energy metabolism such as food intake, body temperature, energy expenditure, and physical activity were examined. Daily food intake was similar between WT and SIRT1 LKO mice on a regular chow diet. No significant difference in body temperature was noted at 10 AM and 5 PM (Fig. 6D). Comprehensive metabolic cage studies indicated that the rates of oxygen consumption (VO2) and carbon dioxide production (VCO2) were reduced in SIRT1 LKO mice under feeding conditions. The calculated energy expenditure was reduced by 20% and 23% in SIRT1 LKO mice during the light and dark phases respectively. Similarly, the rates of VO2, VCO2, and energy expenditure were much lower in SIRT1 LKO mice than those in WT mice during a 24-h fast through light and dark cycles, although metabolic rates were markedly decreased in both genotypes of mice in the fasting state. Moreover, the respiratory quotients (RQ = VCO2/VO2) appeared indistinguishable in WT and SIRT1 LKO mice in fed and fasted states, likely due to the combined decrease in both VO2 and VCO2. This may reflect the relatively equal use of carbohydrates versus lipids as an energy source in whole body. Circadian locomotor activity, as measured by ambulatory movement over a 12-h block of light and dark phases, was similar between the two genotypes of mice (Fig. 6E and F). Collectively, the obesity-prone phenotypes of SIRT1 LKO mice were characterized by an elevation in fat mass, a reduction in energy expenditure, but no significant alterations in lean mass, food intake, body temperature, or physical activity.
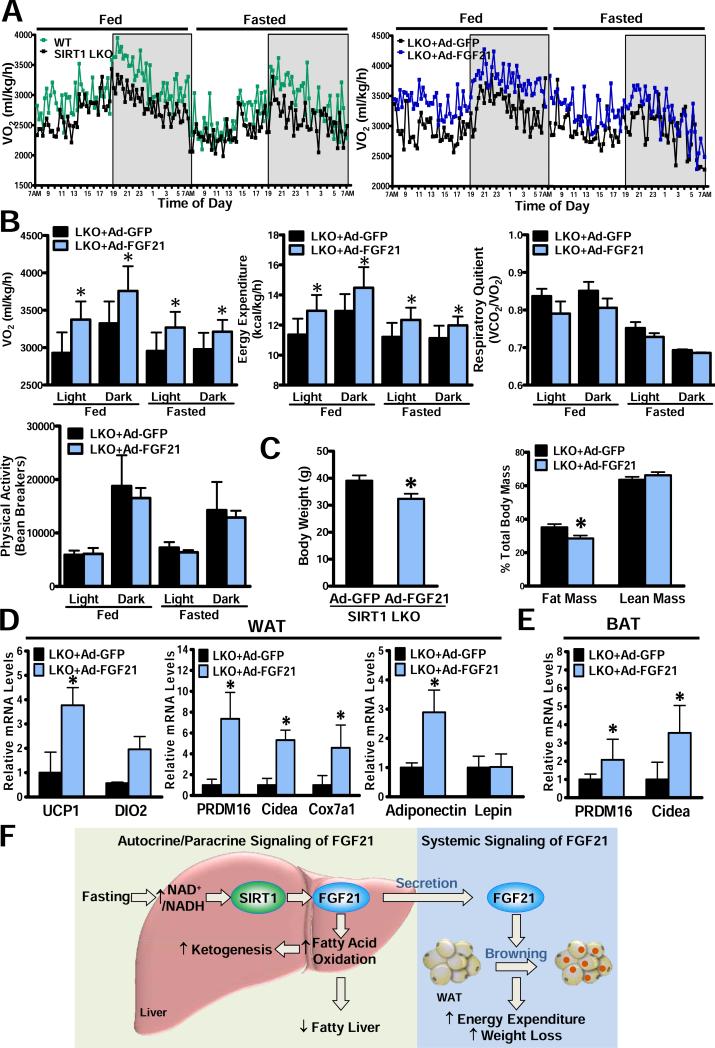
Adenoviral delivery of FGF21 into SIRT1 LKO mice increases energy expenditure and promotes “browning” of white adipose tissue
To directly assess whether suppression of FGF21 is responsible for energy metabolic disturbances in SIRT1 LKO mice, indirect calorimetry was examined in a cohort of SIRT1 LKO mice at two weeks post-injection of either Ad-GFP or Ad-FGF21. As shown in Fig. 7A-C, hepatic overexpression of FGF21 caused a 15% and 10% increase in VO2 consumption in SIRT1 LKO mice under feeding conditions throughout the light and dark cycles. Hepatic overexpression of FGF21 normalized the decreased energy expenditure in SIRT1 LKO mice without significantly affecting RQ or locomotor activity. Reactivation of FGF21 lowered body weight in SIRT1 LKO mice with an approximately 10% reduction in fat mass and no change in lean mass. These data indicate that FGF21 may play an important role in mediating the effect of hepatic SIRT1 on energy expenditure and adiposity.
Fig. 7. Hepatic overexpression of FGF21 rescues the deregulation of whole-body energy metabolism and promotes “browning” of WAT in SIRT1 LKO mice.
A. Circadian changes in VO2 are measured by indirect calorimetry over 48 h in mice in fed and fasted states. B. The rates of VO2 and energy expenditure are elevated by hepatic overexpression of FGF21 in SIRT1 LKO mice in fed and fasted states through light and dark cycles (n = 4). RQ and locomotor activity are similar between the two genotypes of mice. C. Body weight and body composition in mice (n = 4-7). D and E. Adenoviral delivery of FGF21 increases the transcription of brown-fat-like genes and adiponectin in WAT (D) and BAT (E) of SIRT1 LKO mice; *P<0.05, vs. Ad-GFP-injected SIRT1 LKO mice. F. The proposed model for nutrient regulation of FGF21 through SIRT1. Prolonged fasting increases NAD+/NADH ratio and stimulates hepatic SIRT1, which in turn promotes expression, production and release of hepatic FGF21. Induction of FGF21 by hepatic SIRT1 stimulates fatty acid oxidation and ketogenesis, enhances metabolic adaptation to fasting, and improves fatty liver disease. Activation of SIRT1-FGF21 cascade may represent a liver-adipose tissue axis that contributes to reduced adiposity and body weight by promoting white fat “browning” and increasing energy expenditure.
Because FGF21 has emerged as a regulator of brown-fat-like features in white adipose tissue (WAT)23, we further investigated whether enhanced energy expenditure by FGF21 treatment in SIRT1 LKO mice could be attributed to altered transcription of key genes related to the “browning” program in WAT or thermogenic genes in brown adipose tissue (BAT). Strikingly, mRNA amounts of genes that govern the “browning” of WAT, such as UCP1 and the BAT program coactivator PRDM16, as well as expression of other BAT markers, DIO2, Cidea, and Cox7a1, were increased 2- to 6-fold in WAT of SIRT1 LKO mice injected with Ad-FGF21, suggesting that elevated circulating FGF21 in vivo promotes development of brown fat-like characteristics in WAT (Fig. 7D). Notably, mRNA amounts of other white adipose genes such as adiponectin, but not leptin, were also increased (Fig. 7E). Ad-FGF21 treatment appeared to enhance BAT activity, since expression of PRDM16 and Cidea in BAT was stimulated. Collectively, activation of SIRT1-FGF21 signaling represents an important liver-adipose tissue axis that stimulates the “browning” of WAT and acts to maintain systemic energy balance.
Discussion
The characterization of phenotypic changes of SIRT1 LKO mice, such as hepatic steatosis, increased adiposity, and reduced whole-body oxygen consumption, establishes SIRT1 as a novel upstream regulator of hormone FGF21 for the autocrine/paracrine modulation of hepatic fatty acid metabolism as well as the endocrine regulation of WAT “browning” and whole-body energy homeostasis. Hepatic SIRT1 deficiency results in diminished hepatic production and release of FGF21, impaired β-oxidation, stimulated lipogenesis, and reduced energy expenditure. The discovery of SIRT1-dependent induction of FGF21 is also supported by the fact that hepatic steatotic and obesity-prone phenotypes in SIRT1 LKO mice can be rescued by reactivation of FGF21. Fig. 7F depicts the novel function of FGF21 as a downstream mediator of local and systemic effects of hepatic SIRT1 on hepatic steatosis, white fat “browning”, energy homeostasis, and body weight control.
Regulation of FGF21 by SIRT1 in the liver
The most important implication of the present study is the identification of novel cross-talk between nutrient sensors SIRT1 and FGF21. Several lines of evidence have suggested similar properties of SIRT1 and FGF21. First, hepatic SIRT1 activity is induced by prolonged fasting via the increased NAD+/NADH ratio. Hepatic expression of FGF21 is also stimulated by fasting via PPARα9. Second, SIRT1 gain-of-function in SirBACO mice is protective against obesity-induced hepatic steatosis and glucose intolerance18. Likewise, transgenic mice overexpressing FGF21 in the liver are resistant to diet-induced obesity and insulin resistance8. Pharmacological administration of FGF21 to ob/ob or db/db mice attenuates hepatic steatosis and enhances insulin sensitivity8, 12. Third, the metabolic defects seen in SIRT1 LKO mice are similar to those observed in FGF21 KO mice21. Fasting-induced hepatic gene expression and protein secretion of FGF21 are dramatically repressed in SIRT1 LKO mice. Metabolic defects of the adaptive response to fasting seen in SIRT1 LKO mice are reconstituted by hepatic overexpression of FGF21. In further support of the notion that FGF21 is necessary for upregulation of β-oxidation by SIRT1, our in vitro studies and others’17 indicate that SIRT1 induces gene and protein expression of FGF21 in hepatocytes. Importantly, the ability of SIRT1 to stimulate CPT-1α expression is abrogated by FGF21 knockdown or treatment with the inhibitor FGF21 N17. Since FGF21 is identified as a target gene of nuclear receptors, RARβ and PPARα9, 20, it is possible that SIRT1 integrates with nuclear receptors for transcriptional regulation of FGF21 at multiple levels.
Ablation of SIRT1 in the liver increases the propensity for hepatic steatosis and late-onset obesity by suppressing FGF21 production
One major finding of the present study is that pronounced hepatic steatosis is developed in SIRT1 LKO mice and can be attributed at least in part to suppression of FGF21, fatty acid oxidation, and ketogenesis along with stimulation of lipogenic genes in liver. These results are consistent with hepatic lipid accumulation seen in SIRT1 LKO mice challenged with high-fat diets17. Even though FGF21, in the setting of fasting, is not required for the inhibition of SREBP-1 by SIRT1, our data could not rule out the possibility that FGF21, in the setting of hyperinsulinemia and obesity, may suppress SREBP-1. The importance of FGF21 in SIRT1 actions is further supported likely by the fact that gain-of-function of FGF21 protects against hepatic steatosis and obesity-prone phenotypes in SIRT1 LKO mice by increasing hepatic fatty acid oxidation and whole body energy expenditure. Since the alleles of two common SIRT1 variants are associated with a higher risk of being overweight or obese in humans24, our studies may provide mechanistic insight into a role of defective hepatic SIRT1 in triggering FGF21 impairment and energy metabolic perturbation in overweight individuals. Our studies support the rationale for targeting FGF21 as novel therapy to treat humans with obesity associated with SIRT1 dysfunction.
Regulation of “Browning” of white adipose tissue by SIRT1-dependent induction of FGF21
The data presented here strongly suggest that induction of FGF21 by SIRT1 contributes to enhanced energy expenditure and weight loss likely through white fat “browning”, because gain-of-function of FGF21 in SIRT1 LKO mice stimulates expression of “browning” related genes such as UCP1 and PRDM16. In support of our findings, recent studies by the Shulman group also demonstrated that FGF21 administration increases WAT “browning” and energy expenditure in high fat-fed mice. This underlying mechanism may also explain the increased energy expenditure seen in SIRT1 transgenic mice18. Interestingly, increased expression of adiponectin in WAT is evident in mice injected with Ad-FGF21, which is consistent with recent studies that FGF21 stimulates adiponectin production in WAT and has a therapeutic impact on metabolic disorders25. Promotion of white fat “browning” by targeting the SIRT1-FGF21 axis represents an attractive, therapeutic approach to combat the obesity epidemic.
In conclusion, the present study demonstrates that the loss of hepatic SIRT1 reduces hepatic and circulating FGF21 levels and subsequently inhibits hepatic β-oxidation and energy expenditure. The convergence of these defects exacerbates hepatic steatosis and ultimately leads to increased weight gain. SIRT1 is an endogenous activator of FGF21 in hepatocytes that transduces fasting signals to the stimulation of hepatic β-oxidation and ketogenesis as well as systemically controls WAT “browning” and energy homeostasis. This SIRT1-FGF21 axis represents a novel hepatocyte-derived endocrine signaling to potentially combat hepatic steatosis and obesity in humans.
Supplementary Material
Acknowledgments
We greatly appreciate Dr. Richard A. Cohen for helpful discussion. We are grateful to Dr. Francesca Seta for technical assistance in gene chip assays and Dr. Markus Michael Bachschmid for a gift of SIRT1 vector.
Funding: This work is supported by the National Institutes of Health Grants RO1 DK076942 and R21 AA021181, Robert Dawson Evans Faculty Merit Award, and Wing Tat Lee Award (to M. Zang), and by Boston University CTSI subsidy fund UL1RR025771 (to Y. Li).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contribution: Y.L., and M.Z., contributed to experiment design; Y.L., K.W., A.G., J.L., J.J. contributed to the acquisition and analysis of data; A.C.A., A.K., and G.L. provided reagents and material support; Q.Y., B.G., and G.L. reviewed the manuscript; M.Z. obtained the funding and wrote the manuscript.
The authors disclose no conflicts of interest.
References
- 1.Cahill GF. Fuel metabolism in starvation. Annual Review of Nutrition. 2006;26:1–22. doi: 10.1146/annurev.nutr.26.061505.111258. [DOI] [PubMed] [Google Scholar]
- 2.Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, Lan F, Walsh K, Wierzbicki M, Verbeuren TJ, Cohen RA, Zang M. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem. 2008;283:20015–20026. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerhart-Hines Z, Rodgers JT, Bare O, Kim CLSH, Kim SH, Mostoslavsky R, Alt FW, Wu ZD, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1 alpha. Embo Journal. 2007;26:1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ponugoti B, Kim DH, Xiao Z, Smith Z, Miao J, Zang MW, Wu SY, Chiang CM, Veenstra TD, Kemper JK. SIRT1 Deacetylates and Inhibits SREBP-1C Activity in Regulation of Hepatic Lipid Metabolism. Journal of Biological Chemistry. 2010;285:33959–33970. doi: 10.1074/jbc.M110.122978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zang MW, Xu SQ, Maitland-Toolan KA, Zuccollo A, Hou XY, Jiang BB, Wierzbicki M, Verbeuren TJ, Cohen RA. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006;55:2180–2191. doi: 10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Xu SQ, Mihaylova MM, Zheng B, Hou XY, Jiang BB, Park O, Luo ZJ, Lefai E, Shyy JYJ, Gao B, Wierzbicki M, Verbeuren TJ, Shaw RJ, Cohen RA, Zang MW. AMPK Phosphorylates and Inhibits SREBP Activity to Attenuate Hepatic Steatosis and Atherosclerosis in Diet-Induced Insulin-Resistant Mice. Cell Metabolism. 2011;13:376–388. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Xu SQ, Giles A, Nakamura K, Lee JW, Hou XY, Donmez G, Li J, Luo ZJ, Walsh K, Guarente L, Zang MW. Hepatic overexpression of SIRT1 in mice attenuates endoplasmic reticulum stress and insulin resistance in the liver. Faseb Journal. 2011;25:1664–1679. doi: 10.1096/fj.10-173492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB. FGF-21 as a novel metabolic regulator. Journal of Clinical Investigation. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPAR alpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metabolism. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Kharitonenkov A, Larsen P. FGF21 reloaded: challenges of a rapidly growing field. Trends Endocrinol Metab. 2011;22:81–86. doi: 10.1016/j.tem.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Kharitonenkov A, Dunbar JD, Bina HA, Bright S, Moyers JS, Zhang C, Ding L, Micanovic R, Mehrbod SF, Knierman MD, Hale JE, Coskun T, Shanafelt AB. FGF-21/FGF-21 receptor interaction and activation is determined by beta Klotho. Journal of Cellular Physiology. 2008;215:1–7. doi: 10.1002/jcp.21357. [DOI] [PubMed] [Google Scholar]
- 12.Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li YS, Lindberg RA, Chen JL, Jung DY, Zhang ZY, Ko HJ, Kim JK, Veniant MM. Fibroblast Growth Factor 21 Reverses Hepatic Steatosis, Increases Energy Expenditure, and Improves Insulin Sensitivity in Diet-Induced Obese Mice. Diabetes. 2009;58:250–259. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, Hansen BC, Shanafelt AB, Etgen GJ. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007;148:774–781. doi: 10.1210/en.2006-1168. [DOI] [PubMed] [Google Scholar]
- 14.Adams AC, Coskun T, Irizarry Rovira AR, Schneider MA, Raches DW, Micanovic R, Bina HA, Dunbar JD, Kharitonenkov A. Fundamentals of FGF19 & FGF21 Action In Vitro and In Vivo. Plos One. 2012;7:e38438. doi: 10.1371/journal.pone.0038438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Camporez JP, Jornayvaz FR, Petersen MC, Pesta D, Guigni BA, Serr J, Zhang D, Kahn M, Samuel VT, Jurczak MJ, Shulman GI. Cellular Mechanisms by Which FGF21 Improves Insulin Sensitivity in Male Mice. Endocrinology. 2013;154:3099–3109. doi: 10.1210/en.2013-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaich G, Chien JY, Fu H, Glass LC, Deeg MA, Holland WL, Kharitonenkov A, Bumol T, Schilske HK, Moller DE. The Effects of LY2405319, an FGF21 Analog, in Obese Human Subjects with Type 2 Diabetes. Cell Metab. 2013;18:333–340. doi: 10.1016/j.cmet.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo XM, Li XL. Hepatocyte-Specific Deletion of SIRT1 Alters Fatty Acid Metabolism and Results in Hepatic Steatosis and Inflammation. Cell Metabolism. 2009;9:327–338. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banks AS, Kon N, Knight C, Matsumoto M, Gutierrez-Juarez R, Rossetti L, Gu W, Accili D. SirT1 Gain of Function Increases Energy Efficiency and Prevents Diabetes in Mice. Cell Metabolism. 2008;8:333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen D, Bruno J, Easlon E, Lin SJ, Cheng HL, Alt FW, Guarente L. Tissue-specific regulation of SIRT1 by calorie restriction. Genes & Development. 2008;22:1753–1757. doi: 10.1101/gad.1650608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Wong K, Walsh K, Gao B, Zang MW. Retinoic Acid Receptor beta Stimulates Hepatic Induction of Fibroblast Growth Factor 21 to Promote Fatty Acid Oxidation and Control Whole-body Energy Homeostasis in Mice. Journal of Biological Chemistry. 2013;288:10490–10504. doi: 10.1074/jbc.M112.429852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Badman MK, Koester A, Flier JS, Kharitonenkov A, Maratos-Flier E. Fibroblast Growth Factor 21-Deficient Mice Demonstrate Impaired Adaptation to Ketosis. Endocrinology. 2009;150:4931–4940. doi: 10.1210/en.2009-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gesta S, Bezy O, Mori MA, Macotela Y, Lee KY, Kahn CR. Mesodermal developmental gene Tbx15 impairs adipocyte differentiation and mitochondrial respiration. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:2771–2776. doi: 10.1073/pnas.1019704108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, Spiegelman BM. FGF21 regulates PGC-1 alpha and browning of white adipose tissues in adaptive thermogenesis. Genes & Development. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zillikens MC, van Meurs JBJ, Rivadeneira F, Amin N, Hofman A, Oostra BA, Sijbrands EJG, Wittentan JCM, Pols HAP, van Duijn CM, Uitterlinden AG. SIRT1 Genetic Variation Is Related to BMI and Risk of Obesity. Diabetes. 2009;58:2828–2834. doi: 10.2337/db09-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holland WL, Adams AC, Brozinick JT, Bui HH, Miyauchi Y, Kusminski CM, Bauer SM, Wade M, Singhal E, Cheng CC, Volk K, Kuo MS, Gordillo R, Kharitonenkov A, Scherer PE. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 2013;17:790–797. doi: 10.1016/j.cmet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.