Figure 1.
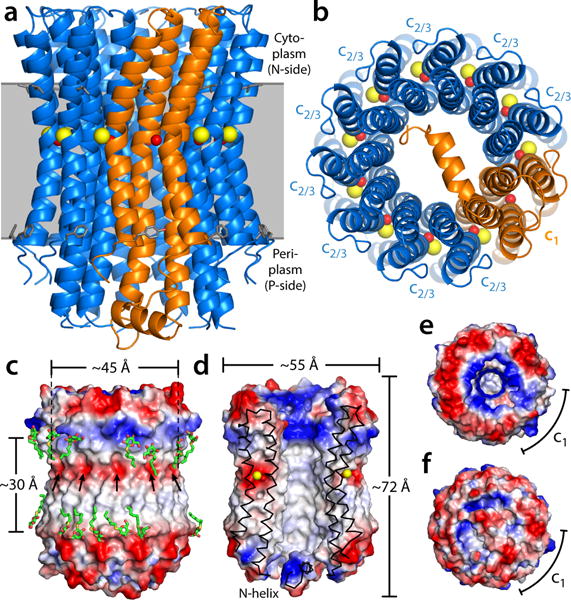
Structure of the heteromeric c-ring from the Acetobacterium woodii ATP synthase. The ring is viewed (a) along the membrane plane and (b) from the periplasm, highlighting the two c-subunit topologies (blue and orange cartoons), as well as the bound Na+ ions (yellow spheres) and the co-coordinating water molecules (red spheres). Note the site within subunit c1 does not bind Na+. Residues L55/F77 in c2/3, and L72/L155/F94/Y177 in c1 indicate the likely position of the ring within the membrane (gray). (c) Electrostatic potential at the outer surface of the c-ring, and (d) at the surface of the central hydrophobic pore. Detergent molecules (green) resolved in the electron density map are highlighted. Arrows indicate the position of the ion-binding sites. (e) Asymmetry of the electrostatic potential on the cytoplasmic face of the c-ring, where the central stalk binds. (f) The N-terminal extension of subunit c1 occludes the central pore almost completely.
