Figure 7.
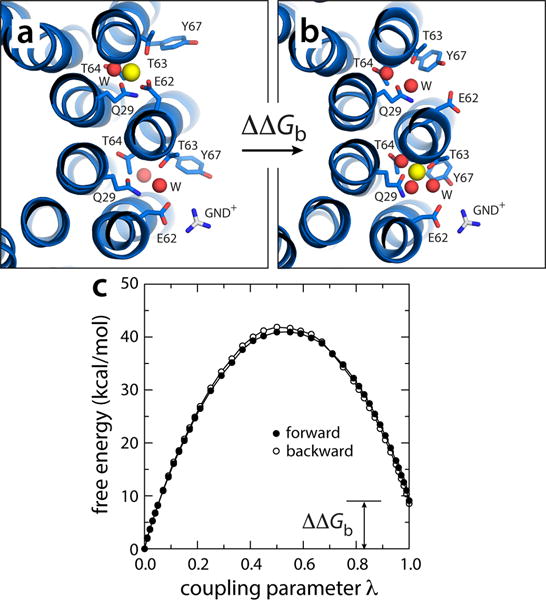
Electrostatic barrier between the P and N-channels. (a) A snapshot was extracted from one of the simulations in which Na+ is spontaneously released (Fig. 5), and the exposed glutamate side-chain was paired to a guanidinium ion (GND+), modeled in to represent the interaction with the key arginine side-chain on TM4 of subunit-a. (b) The free-energy cost of transferring a bound Na+ from the adjacent site, counter-clockwise, to the site engaged to the GND+ ion, was then computed, by gradually decoupling the ion from its environment in configuration (a) and re-coupling it in configuration (b). (c) Free-energy change as a function of the (de)coupling parameter λ. The transfer free energy was calculated in both directions. The c-ring is represented as in Fig. 1. Side-chains and water molecules in the site are highlighted (sticks, spheres). Hydrogen atoms as well as other protein side-chains and all MPD/water molecules are omitted for clarity.
