Abstract
Background
There is limited evidence that among HIV-infected patients haemoglobin A1c (HbA1c) values may not accurately reflect glycaemia. We assessed HbA1c discordance (observed HbA1c − expected HbA1c) and associated factors among HIV-infected participants in the Multicenter AIDS Cohort Study (MACS).
Methods
Fasting glucose (FG) and HbA1c were measured at each semi-annual MACS visit since 1999. All HIV-infected and HIV-uninfected men for whom at least one FG and HbA1c pair measurement was available were evaluated. Univariate median regression determined the association between HbA1c and FG by HIV serostatus. The relationship between HbA1c and FG in HIV-uninfected men was used to determine the expected HbA1c. Generalized estimating equations determined factors associated with the Hb1Ac discordance among HIV-infected men. Clinically significant discordance was defined as observed HbA1c − expected HbA1c ≤−0.5%.
Results
Over 13 years, 1500 HIV-uninfected and 1357 HIV-infected men were included, with a median of 11 visits for each participant. At an FG of 125 mg/dL, the median HbA1c among HIV-infected men was 0.21% lower than among HIV-uninfected men and the magnitude of this effect increased with FG >126 mg/dL. Sixty-three percent of HIV-infected men had at least one visit with clinically significant HbA1c discordance, which was independently associated with: low CD4 cell count (<500 cells/mm3); a regimen containing a protease inhibitor, a non-nucleoside reverse transcriptase inhibitor or zidovudine; high mean corpuscular volume; and abnormal corpuscular haemoglobin.
Conclusion
HbA1c underestimates glycaemia in HIV-infected patients and its use in patients with risk factors for HbA1c discordance may lead to under-diagnosis and to under-treatment of established diabetes mellitus.
Keywords: HbA1c, diabetes, mean corpuscular volume, glycosylated haemoglobin, HIV
Introduction
Use of combined antiretroviral therapy (cART) has dramatically improved survival and life expectancy among HIV-infected persons over time.1–3 In this context, the management of chronic comorbidities, including metabolic complications, has become a major healthcare issue. Diabetes mellitus (DM) is common in HIV-infected persons and, as in the general population, is associated with hypertension, dyslipidaemia, cardiovascular disease and renal impairment.4–8 In order to reduce the risk of macrovascular and microvascular complications of DM, adequate glycaemic control is essential.9 Haemoglobin A1c (HbA1c), which measures the percentage of haemoglobin A that is glycated, is the primary index of glycaemic control in patients with DM and has been recommended for the diagnosis of DM.10
Among HIV-infected persons, HbA1c values may not accurately reflect glycaemia. Two recent studies have suggested that HbA1c underestimates mean fasting glucose (FG) among HIV-infected patients, possibly as a result of low-grade haemolysis,11,12 although the clinical significance of this effect is uncertain.13 In these studies, factors associated with HbA1c inaccuracy were higher mean corpuscular volume (MCV) as well as low serum haptoglobin, use of certain antiretroviral drugs (especially zidovudine or abacavir) or medication to treat diabetes.
Using data from the Multicenter AIDS Cohort Study (MACS) collected between 1 April 1999 and 31 March 2012, we sought to determine whether HbA1c underestimated FG in HIV-infected persons compared with HIV-uninfected persons, whether the magnitude of HbA1c discordance was clinically significant and what factors were associated with HbA1c discordance.
Methods
Study population
The MACS is an ongoing prospective study of HIV-1 infection among men who have sex with men (MSM) at four centres in the USA: Chicago, Baltimore/Washington DC, Pittsburgh and Los Angeles. Institutional review boards at each site approved the MACS protocol and forms, and each participant gave written informed consent. Details of the study design and follow-up methods have been published.14 Briefly, participants attend semi-annual study visits, which include a detailed interview, physical examination and collection of biological specimens.
Beginning in April 1999, a fasting (≥8 h) serum sample was obtained, from which glucose and HbA1c were measured. Our study population consisted of all MACS participants for whom at least one FG and HbA1c pair measured at the same visit was available. The first visit at which an FG–HbA1c pair was available was defined as the baseline visit and all subsequent available FG–HbA1c pairs from a participant were used in analyses. We excluded from our analysis those visits at which non-FG levels were obtained.
Laboratory methods
FG levels were measured by the combined hexokinase/glucose-6-phosphate dehydrogenase method at a central laboratory (Heinz Laboratory, Pittsburgh, PA), (coefficient of variation 1.8%). HbA1c and complete blood counts (CBCs) were measured by Quest Diagnostics (Baltimore, MD) using standard clinical assays. HbA1c was measured by immunoassay using the Roche Cobas Integra 800 analytical system (Indianapolis, IN; coefficient of variation <3.3%). CBC was measured using a Coulter LH 750 (Beckman Coulter, Inc., Brea, CA). Haemoglobin, MCV and mean corpuscular haemoglobin (MCH) were examined since recent studies have identified relationships with HbA1c discordance.11,12,15 Standardized protocols were used to measure T lymphocyte subsets.16 Plasma HIV-1 RNA was assessed using either the Roche standard assay or the Roche ultrasensitive assay.
Statistical analysis
Univariate median regression with HbA1c as the dependent variable was used to explore the association between FG and HbA1c by HIV status. The regression was done separately for FG values below and above 126 to assess differences in associations with HbA1c across the glycaemic spectrum. This was done by including interaction terms between indicators for FG ≥126 mg/dL, FG <126 mg/dL and HIV in the model. Because participants contributed multiple FG and HbA1c measurements to analyses, results were bootstrapped to assure appropriate 95% CIs.
Multivariate median regression was then used among the HIV-uninfected person-visits to predict the expected HbA1c values among the HIV-infected participants at each person-visit. The relationship between HbA1c and FG (centred at 125 mg/dL) was adjusted for age (centred at 50 years) and race [white (reference group), black, Hispanic or other race]. Then the expected HbA1c was subtracted from the observed HbA1c value for each HIV-infected person-visit to obtain the HbA1c discordance. This discordance value was used as the primary outcome in a linear mixed effects regression analysis to determine factors associated with HbA1c discordance, with a random intercept to account for the repeated outcome measurements. Potential explanatory variables examined included age, race, BMI [<18.5, 18.5–24.9 (reference group), 25–29, ≥30], CD4 cell count [<200, 200–349, 350–499, ≥500 cells/mm3 (reference group)], current ART use and plasma HIV-1 RNA level [no ART (reference group), on ART with or without RNA ≤ 400 copies/mL], hepatitis C virus (HCV) infection (defined by positive antibody or HCV RNA, yes/no), a history of clinical AIDS (yes/no),17 use of individual classes of ARTs [protease inhibitors (PIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), use of selected individual nucleoside reverse transcriptase inhibitors (NRTIs), including zidovudine, lamivudine, emtricitabine, abacavir or tenofovir, in the past 6 months], as well as haemoglobin level [stratified in categories of <10, 10–11, 12–13, ≥14 mg/dL (reference group)], MCV [<85 (reference group), 85–89, 90–94, 95–99, 100–104, ≥105 fL] and MCH [<27, 27–31 (reference group), >31 pg].
A second outcome was based upon whether the HbA1c discordance was ≤−0.5% (yes versus no), which is considered clinically significant,18 and analysed by logistic regression models, with a generalized estimating equation to account for within-subject correlation.
Missing data for BMI (9.2% of 15 161 HIV-infected person-visits), CD4 cell count (1.6%), HIV-1 RNA (0.6%), haemoglobin (1.4%), MCV (1.1%) and MCH (1.2%) were extrapolated based on the longitudinal data for each participant. All statistical analyses were performed using SAS software, version 9.2 (SAS Institute Inc., Cary, NC).
Results
Baseline characteristics
Of the 3244 men with a visit between 1 April 1999 and 31 March 2012, 2857 (1500 HIV-uninfected and 1357 HIV-infected participants) had at least one visit with both FG and HbA1c measurements (Table 1). The mean of number of visits for each participant was 11 (range 1–26). Compared with the HIV-uninfected men, HIV-infected men were younger, were more likely to be Caucasian and have a lower level of education and BMI and were more likely to have DM.
Table 1.
Baseline characteristics of 2857 MACS participantsa
| HIV-uninfected (n = 1500) | HIV-infected (n = 1357) | |
|---|---|---|
| Age (years), median (IQR) | 46 (40–53) | 43 (37–49) |
| Race, n (%) | ||
| Caucasian | 1019 (68) | 737 (54) |
| African American | 334 (22) | 400 (30) |
| Hispanic or other race | 147 (10) | 220 (16) |
| Education, n (%) | ||
| college degree or above | 852 (57) | 569 (42) |
| BMI, median (IQR) | 26 (23–29) | 25 (23–27) |
| Current diabetes, n (%)b | 75 (5) | 102 (8) |
| Fasting glucose (mg/dL), median (IQR) | 90 (82–98) | 91 (83–99) |
| HbA1c, (%), median (IQR) | 5.2 (4.9–5.5) | 5.0 (4.6–5.4) |
| Haemoglobin (g/dL), median (IQR) | 15 (14.4–15.7) | 14.7 (13.9–15.6) |
| MCV (fL), median (IQR) | 91 (88–94) | 100 (91–111) |
| MCH (pg), median (IQR) | 31 (30–32) | 34 (31–38) |
| CD4 cell count (cells/mm3), median (IQR) | — | 486 (320–679) |
| ART treated, with HIV RNA <400 copies/mL, n (%) | — | 770 (76%) |
| HCV infected, n (%)c | 73 (5) | 148 (11) |
| HBV infected, n (%)d | 31 (2) | 88 (7) |
| Treatment exposure, n (%) | ||
| ART naive | 254 (19) | |
| past ART | 95 (7) | |
| current ART (in last 6 months) | — | 1008 (74) |
| PI-containing regimen (%) | — | 59 |
| NNRTI-containing regimen (%) | — | 47 |
| ZDV-containing regimen (%) | — | 41 |
| ABC-containing regimen (%) | — | 25 |
| 3TC-containing regimen (%) | — | 72 |
| TDF-containing regimen (%) | — | 18 |
ZDV, zidovudine; ABC, abacavir; 3TC, lamivudine; TDF, tenofovir.
aBaseline was the first visit with both fasting glucose and HbA1c data from visit 31 onwards.
bHistory of diabetes was defined as a fasting glucose ≥126 mg/dL or (diagnosed with diabetes and use of medications) at any visit before or at baseline visit.
cHCV infection was defined as having a positive serum antibody to HCV or positive HCV RNA at baseline visit.
dHepatitis B virus (HBV) infection was defined as having a positive serum HBV surface antigen test at the first visit or during MACS follow-up visits.
The median FG was higher in the HIV-infected group at the index visit than in the HIV-uninfected group (91 and 90 mg/dL, respectively; P = 0.013), whereas the HbA1c level was lower (5.0% and 5.2%, respectively; P < 0.001). The HIV-infected men had a higher median MCV (P < 0.001), higher median MCH (P < 0.001) and lower median haemoglobin (P < 0.001) than the HIV-uninfected men.
Relationship between HbA1c and FG in HIV-infected and HIV-uninfected men
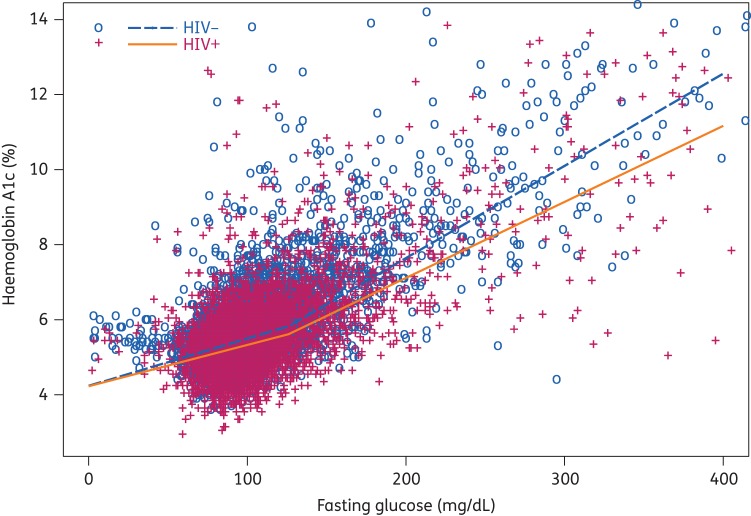
The median HbA1c at FG = 125 mg/dL was 5.82% in the HIV-uninfected group compared with 5.61% in the HIV-infected group (median HbA1c discordance −0.21; P < 0.001) (Figure 1). The magnitude of the difference in the slopes of HbA1c versus FG in HIV-infected and uninfected men was statistically significant for FG ≥ 126 mg/dL (difference in slope −0.0044% for each additional mg/dL; P = 0.018). The slopes, however, were not significantly different for FG < 125 mg/dL (P = 0.094).
Figure 1.
Relationship between haemoglobin A1c and fasting glucose in HIV-infected and HIV-uninfected men using median regression with a knot at 125 mg/dL.
Factors associated with HbA1c discordance among HIV-infected men
Among the HIV-infected men, the median HbA1c discordance (difference between observed and expected HbA1c values) was −0.17%, with the 25th percentile of −0.49 and the 75th percentile of 0.11. Of these person-visits (n = 14 860), 24.5% had an HbA1c discordance ≤−0.5%, occurring in 871/1378 (63%) men.
In univariate models (Table 2), a lower than expected HbA1c value (observed − expected <0) was associated with a CD4 cell count <500 cells/mm3, use of a PI, zidovudine and/or a lamivudine-containing regimen and higher MCV and MCH. Older age, non-Caucasian race and obesity were associated with a higher than expected HbA1c (observed − expected >0).
Table 2.
Parameter estimates (95% CIs) from linear mixed models of discordance (observed − expected HbA1c) among HIV-infected patients
| Factors | Univariate model | P value | Multivariate Model 1 | P value | Multivariate Model 2 | P value | Multivariate Model 3 | P value | Multivariate Model 4 | P value |
|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | −0.123 (−0.149, −0.096) | <0.001 | −0.295 (−0.33, −0.259) | <0.001 | −0.114 (−0.161, −0.067) | <0.001 | 0.176 (0.112, 0.24) | <0.001 | 0.072 (−0.033, 0.177) | 0.178 |
| Age (per 1 year) | 0.024 (0.022, 0.026) | <0.001 | 0.025 (0.023, 0.027) | <0.001 | 0.027 (0.025, 0.029) | <0.001 | 0.017 (0.015, 0.019) | <0.001 | 0.015 (0.012, 0.017) | 0.000 |
| Race (versus white) | ||||||||||
| black | 0.145 (0.095, 0.194) | <0.001 | 0.256 (0.199, 0.313) | <0.001 | 0.268 (0.209, 0.327) | <0.001 | 0.131 (0.078, 0.183) | <0.001 | 0.091 (0.032, 0.15) | 0.003 |
| Hispanic or other | 0.122 (0.059, 0.184) | <0.001 | 0.364 (0.292, 0.436) | <0.001 | 0.384 (0.311, 0.457) | <0.001 | 0.234 (0.169, 0.298) | <0.001 | 0.197 (0.126, 0.268) | 0.000 |
| BMI (kg/m2, versus 18.5–24.9) | ||||||||||
| <18.5 | 0.05 (−0.04, 0.139) | 0.275 | 0.014 (−0.074, 0.102) | 0.757 | 0.012 (−0.076, 0.1) | 0.791 | 0.012 (−0.072, 0.096) | 0.782 | 0.047 (−0.044, 0.138) | 0.312 |
| 25–29 | 0.044 (0.016, 0.072) | 0.002 | 0.034 (0.006, 0.062) | 0.018 | 0.027 (−0.001, 0.055) | 0.058 | 0.007 (−0.02, 0.033) | 0.611 | 0.011 (−0.02, 0.042) | 0.481 |
| ≥30 | 0.284 (0.239, 0.329) | <0.001 | 0.246 (0.2, 0.292) | <0.001 | 0.239 (0.193, 0.285) | <0.001 | 0.173 (0.13, 0.216) | <0.001 | 0.181 (0.131, 0.232) | 0.000 |
| Current CD4 cell count (cells/mm3, versus ≥500) | ||||||||||
| <200 | −0.11 (−0.154, −0.065) | <0.001 | −0.115 (−0.161, −0.07) | <0.001 | −0.142 (−0.185, −0.098) | <0.001 | −0.308 (−0.371, −0.245) | 0.000 | ||
| 200–349 | −0.101 (−0.132, −0.07) | <0.001 | −0.093 (−0.124, −0.062) | <0.001 | −0.108 (−0.137, −0.078) | <0.001 | −0.137 (−0.175, −0.099) | 0.000 | ||
| 350–499 | −0.061 (−0.085, −0.037) | <0.001 | −0.06 (−0.084, −0.036) | <0.001 | −0.071 (−0.094, −0.048) | <0.001 | −0.079 (−0.106, −0.052) | 0.000 | ||
| Current ART and HIV RNA level (copies/mL, versus no ART) | ||||||||||
| on ART with HIV RNA ≤400 | −0.077 (−0.107, −0.047) | 0.000 | −0.174 (−0.205, −0.144) | <0.001 | 0.012 (−0.02, 0.043) | 0.471 | 0.148 (0.064, 0.232) | 0.001 | ||
| on ART with HIV RNA >400 | −0.13 (−0.168, −0.093) | 0.000 | −0.138 (−0.174, −0.101) | <0.001 | 0.021 (−0.016, 0.057) | 0.263 | NA | NA | ||
| HCV-infected, yes versus no | −0.005 (−0.068, 0.059) | 0.882 | −0.082 (−0.151, −0.013) | <0.001 | −0.029 (−0.092, 0.034) | 0.363 | 0.016 (−0.056, 0.088) | 0.665 | ||
| AIDS, yes versus no | 0.026 (−0.028, 0.079) | 0.346 | 0.021 (−0.036, 0.079) | 0.466 | 0.02 (−0.033, 0.072) | 0.466 | 0.048 (−0.014, 0.11) | 0.133 | ||
| Haemoglobin (mg/dL, versus ≥14) | ||||||||||
| <10 | 0.045 (−0.071, 0.162) | 0.444 | −0.053 (−0.164, 0.057) | 0.343 | 0.192 (0.054, 0.33) | 0.006 | ||||
| 10–11 | −0.041 (−0.097, 0.015) | 0.148 | −0.095 (−0.148, −0.041) | 0.001 | −0.102 (−0.17, −0.035) | 0.003 | ||||
| 12–13 | 0.013 (−0.011, 0.037) | 0.301 | −0.014 (−0.037, 0.009) | 0.245 | −0.01 (−0.038, 0.018) | 0.499 | ||||
| MCV (fL, versus <85) | ||||||||||
| 85–89 | −0.138 (−0.186, −0.091) | <0.001 | −0.154 (−0.205, −0.103) | <0.001 | −0.113 (−0.188, −0.037) | 0.003 | ||||
| 90–94 | −0.176 (−0.224, −0.127) | <0.001 | −0.193 (−0.248, −0.138) | <0.001 | −0.157 (−0.236, −0.078) | 0.000 | ||||
| 95–99 | −0.273 (−0.323, −0.223) | <0.001 | −0.283 (−0.344, −0.222) | <0.001 | −0.228 (−0.312, −0.143) | 0.000 | ||||
| 100–104 | −0.432 (−0.484, −0.379) | <0.001 | −0.422 (−0.486, −0.358) | <0.001 | −0.349 (−0.436, −0.261) | 0.000 | ||||
| ≥105 | −0.673 (−0.723, −0.624) | <0.001 | −0.616 (−0.679, −0.553) | <0.001 | −0.518 (−0.608, −0.428) | 0.000 | ||||
| MCH (pg, versus 27–31) | ||||||||||
| <27 | 0.073 (−0.007, 0.154) | 0.074 | −0.077 (−0.163, 0.01) | 0.084 | −0.091 (−0.201, 0.02) | 0.108 | ||||
| >31 | −0.256 (−0.28, −0.232) | 0.000 | −0.064 (−0.095, −0.032) | <0.001 | −0.084 (−0.123, −0.044) | 0.000 | ||||
| PI, yes versus no | −0.095 (−0.126, −0.064) | <0.001 | −0.12 (−0.156, −0.084) | 0.000 | ||||||
| NNRTI, yes versus no | 0.033 (0.003, 0.063) | 0.033 | −0.071 (−0.105, −0.037) | 0.000 | ||||||
| ZDV, yes versus no | −0.404 (−0.436, −0.372) | <0.001 | −0.108 (−0.148, −0.068) | 0.000 | ||||||
| 3TC, yes versus no | −0.263 (−0.289, −0.238) | <0.001 | 0.03 (−0.006, 0.066) | 0.106 | ||||||
| FTC, yes versus no | 0.301 (0.276, 0.326) | <0.001 | 0.045 (0.006, 0.085) | 0.025 | ||||||
| ABC, yes versus no | 0.028 (−0.006, 0.062) | 0.109 | 0.04 (0.005, 0.075) | 0.027 | ||||||
| TDF, yes versus no | 0.299 (0.274, 0.324) | <0.001 | 0.023 (−0.011, 0.058) | 0.188 | ||||||
ZDV, zidovudine; 3TC, lamivudine; FTC, emtricitabine; ABC, abacavir; TDF, tenofovir.
The estimate of intercept was for those who were 50 years old in the univariate model and for those who were 50 years old and also in the reference group for other factors in multivariate models.
Factors in multivariate Model 1 included age, race and BMI; factors in multivariate Model 2 included factors in Model 1 and CD4 cell count, current ART and HIV RNA level, HCV and AIDS; factors in multivariate Model 3 included factors in Model 2 and haemoglobin, MCV and MCH; factors in multivariate Model 4 (restricting to those with HIV RNA ≤400 copies/mL) included factors in Model 3 and all ART variables of interest.
HCV infection was defined as having a positive serum antibody to HCV or positive HCV RNA at baseline visit.
We used a series of nested multivariable models to determine the independence of each of these factors in their relationship with HbA1c discordance. In a model that contained demographic and HIV-related parameters (Table 2, Model 2), lower CD4 cell count, ART treatment (with or without a suppressed HIV-1 RNA level) and HCV co-infection were each independently associated with a lower observed than expected HbA1c value. After the addition of haematological parameters (Model 3), lower CD4 cell count, but not current ART or HCV co-infection, remained associated with HbA1c discordance. In addition, HbA1c discordance was associated with abnormal MCH (either above or below the normal range) and higher MCV, with each 5 fL increase in MCV associated with a progressively greater degree of HbA1c discordance. In a final model (Model 4), which included ART variables, we found that ART regimens in the previous 6 months containing a PI, an NNRTI or zidovudine were associated with a lower observed than expected HbA1c value. In contrast, abacavir and emtricitabine were associated with a higher observed HbA1c than expected.
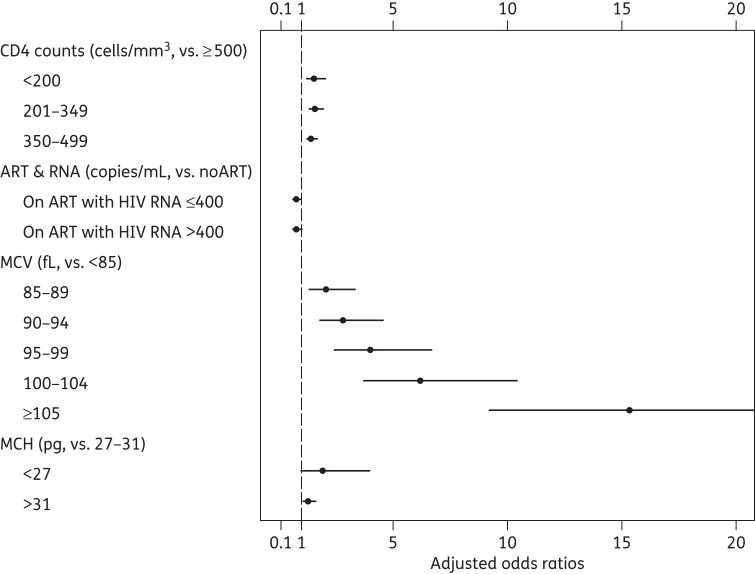
Figure 2 shows the relationship between demographic, HIV-related and haematological factors and the presence of clinically significant HbA1c discordance defined as observed − expected HbA1c ≤−0.5%. In the multivariable logistic model, the odds ratios for the CD4 cell count were similar at 1.57 (95% CI 1.20, 2.07) for CD4 cell count <200 cells/mm3, 1.61 (95% CI 1.31, 1.97) for CD4 209–349 cells/mm3 and 1.45 (95% CI 1.23, 1.70) for CD4 350–499 cells/mm3 versus CD4 >500 cells/mm3 (P < 0.001). Current ART (with or without HIV-1 RNA viral suppression) was not associated with clinically significant HbA1c discordance. The HbA1c discordance increased markedly with each 5 fL increase in MCV, from 2.09 (95% CI 1.31, 3.36 for MCV 85–89 fL) to 15.39 (95% CI 9.19, 25.79 for MCV >105 fL) and was also related to abnormal MCH [1.95 (95% CI 0.95, 4.0) for MCH <27 pg (P = 0.086) versus 1.32 (95% CI 1.05, 1.65) for MCH >31 pg; P = 0.018].
Figure 2.
Factors associated with HbA1c discordance (observed − expected HbA1c) ≤−0.5% among HIV-infected patients in the MACS. Other factors included age, race, BMI, HCV infection, AIDS and haemoglobin. The upper 95% CI for the OR of MCV ≥105 fL was 25.8.
When use of specific ARTs was added to the model, PI use [OR 1.39 (95% CI 1.08, 1.78); P = 0.01] and zidovudine use [OR 1.38 (95% CI 1.06, 1.81); P = 0.019] were independently associated with clinically significant discordance (data not shown). In contrast, abacavir [OR 0.73 (95% CI 0.58, 0.91); P = 0.002], emtricitabine [OR 0.36 (95% CI 0.26, 0.49); P < 0.001] and lamivudine [OR 0.74 (95% CI 0.56, 0.99); P = 0.041] appeared to be protective against clinically significant HbA1c discordance.
Discussion
This analysis of MACS data that span a 13 year period and include 1357 HIV-infected men constitutes the largest study to date that has evaluated whether HbA1c accurately predicts glycaemia among HIV-infected persons. We found that, at an FG of 125mg/dL, median HbA1c values were 0.21% lower in HIV-infected men than in HIV-uninfected men and that the magnitude of this difference increased at higher glucose values. We also found that HbA1c discordance (observed − expected HbA1c) was associated with lower CD4 cell counts, haematological parameters including high MCV and MCH, and exposure to certain ART agents including PIs, NNRTIs and zidovudine. Our findings suggest that HbA1c may not be an adequate marker of glycaemic control in certain HIV-infected persons and that its use may lead to under-treatment of established DM or under-diagnosis of DM when used as a diagnostic criterion.
Following an initial case report,19 three controlled studies have been published to date that addressed the relationship between glycaemia and HbA1c levels in HIV-infected persons. In a retrospective cross-sectional study, Diop et al.11 found that HbA1c underestimated mean FG in HIV-infected patients (n = 112) by ∼12% compared with HIV-uninfected persons. A prospective cross-sectional study12 compared observed and expected HbA1c levels in 100 HIV-infected patients with DM (77% of patients) or fasting hyperglycaemia (23%) and 200 matched HIV-uninfected participants and found that HbA1c underestimated glycaemia (mean of one fasting and one non-fasting sample) among HIV-infected persons by an average of 29 mg/dL. Most recently, Glesby et al.13 compared FG values and HbA1c values among 315 HIV-infected and 109 HIV-uninfected women with DM and found that HbA1c values were modestly lower among HIV-infected women compared with HIV-uninfected women (6.4% and 6.8%, respectively, P = 0.023) at the same level of FG. In our study, we found that, at a glucose level of 125 mg/dL, HbA1c values were a median of 0.21% lower in HIV-infected than in HIV-uninfected men and that the magnitude of this difference increased at higher glucose values. When we examined the HbA1c discordance as a categorical variable we found that 63.2% of men (24.5% observations) had an HbA1c discordance ≤−0.5%, defined as clinically significant.
The mechanisms underlying the underestimation of glycaemia by HbA1c among HIV-infected persons are unclear. Previous reports have suggested that HIV infection and/or its treatment is associated with a low-grade haemolysis,11 thereby leading to a shorter period of time during which haemoglobin within erythrocytes can become glycated. This hypothesis was supported by Diop,11 who found that the difference between measured and predicted HbA1c based on FG was correlated with low serum levels of haptoglobin, a plasma protein that binds to free haemoglobin and decreases during haemolysis.20 In contrast, Kim et al.12 showed no association between haptoglobin and HbA1c discordance in HIV-infected persons. It should be noted that haptoglobin levels might be less reliable indicators of haemolytic anaemia in the setting of inflammation or low-grade extravascular haemolysis. In the current study we did not have measurements of serum haptoglobin, but plan to assess this in future MACS investigations.
As in previous studies, we found that higher MCV was a strong predictor of HbA1c discordance among HIV-infected persons.11,12 Macrocytosis is common among HIV-infected persons and has been attributed to certain antiretroviral agents, including zidovudine, stavudine and lamivudine. Macrocytosis has also been associated with lower HIV-1 RNA levels, independently of specific ART.21–23 Our data suggest that clinicians should be particularly cautious about using HbA1c in the management or diagnosis of DM among HIV-infected persons who have an MCV ≥95 fL, for whom the odds of HbA1c discordance is 4–15-fold higher than among persons with MCV <95 fL.
MCH, defined as the average mass of haemoglobin per erythrocyte, was also an important predictor of HbA1c discordance in our study. Low levels are characteristic of iron deficiency anaemia and may also be related to HbA1c. In a study involving 423 women without DM in the general population,15 an increase of 1 pg in MCH corresponded to a decrease of ∼0.03% in HbA1c, independently of haemoglobin levels or other red cell parameters. Since MCH values are readily available from complete blood cell count reports, identification of an HIV-infected person with a high MCH (>31 pg) might serve as an alert that HbA1c levels need to be interpreted with caution.
As in prior studies, we also found that use of certain ART drugs was associated with HbA1c discordance. Many of these effects, including those associated with zidovudine and PI use, remained significantly associated with HbA1c discordance in models adjusted for MCV and other haematological variables, suggesting that ART effects are not fully mediated by their impact upon erythrocyte parameters. In contrast to the study of Kim et al.,12 we did not detect an association between abacavir use and HbA1c discordance.
A novel finding in our analysis is that HbA1c discordance was strongly associated with CD4 counts <500 cells/mm3. We hypothesize that lower CD4 cell counts among HIV-infected patients are associated with chronic monocyte activation, leading to increased activation of the reticulo-endothelial system, erythrocyte phagocytosis, decreased red cell lifespan and consequent lowering of HbA1c levels.24 Of note, the monocyte activation marker sCD163, which is commonly evaluated in studies of HIV-related immune activation, is a haemoglobin–haptoglobin scavenger receptor and a marker specific for the reactive haemophagocytic syndrome.25 Future work in the MACS will investigate associations between HbA1c discordance and sCD163 levels.
We also found that several demographic factors were related to differences between observed and expected HbA1c, including older age and higher BMI. The finding regarding age has also been observed in NHANES III, in which HbA1c rose by ∼0.10% with each decade after age 40,26 which has been attributed to the acceleration of haemoglobin glycation with advancing age.27 In addition, in our study we used FG as our primary measure of glycaemia and hence did not capture post-prandial fluctuations in blood glucose. Since glucose intolerance increases with ageing, older individuals may have greater post-prandial glucose excursions, which would not impact the expected HbA1c levels that we calculated using FG values. Similarly, obese individuals may also experience larger post-prandial glucose excursions, which could lead to higher observed HbA1c levels than expected. Lastly, as in previous studies in the general population, the observed HbA1c was greater than expected among black and Hispanic compared with white participants.26,28,29 Whether these differences are due to differences in glycaemia or non-glycaemic genetic factors is debated.28
Our study has several limitations. We used FG to assess glycaemia rather than seven-point glucose monitoring or glycaemia as assessed by a continuous glucose monitor, which is the gold standard.30 Our study population included relatively few persons with impaired FG or overt diabetes, for whom inaccuracy of HbA1c would have the highest clinical relevance. In addition, without measurements of other indices of haemolysis we were unable to assess the mechanism(s) accounting for HbA1c discordance. Similarly, we did not have other laboratory-based methods to assess glycaemia, including fructosamine or 1,5-anhydroglucitol, which have been proposed as alternatives to HbA1c among HIV-infected persons.12 Finally, our study included only men and cannot be extrapolated to women.
In conclusion, we found that HbA1c underestimated glycaemia in certain HIV-infected men. This effect was particularly pronounced in men with a CD4 cell count <500 cells/mm3, a higher MCV and a high MCH, and in persons receiving ART regimens that included PIs, NNRTIs or zidovudine. In these sub-populations, it would be prudent for clinicians to use direct measures of glycaemia (i.e. fasting glucose or an oral glucose tolerance test) to diagnose diabetes and, for persons with diabetes, to consider a lower HbA1c level treatment target in order to prevent long-term diabetes complications.
Funding
This work was supported by the National Institutes of Health (U01-AI35042, U01-AI35039, U01-AI35040, U01-AI35041, UM1-AI35043, UL1-TR000424). The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the National Cancer Institute (NCI). Targeted supplemental funding for specific projects was also provided by the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute on Deafness and Communication Disorders (NIDCD). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). Web site located at http://www.statepi.jhsph.edu/macs/macs.html.
Transparency declarations
Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS). MACS (Principal Investigators): Johns Hopkins University Bloomberg School of Public Health (Joseph Margolick); Northwestern University (Steven Wolinsky); University of California, Los Angeles (Roger Detels); University of Pittsburgh (Charles Rinaldo); the Center for Analysis and Management of MACS, Johns Hopkins University Bloomberg School of Public Health (Lisa Jacobson).
L. S. reports a scholarship which supported her as a visiting scholar at Northwestern University from the AIDS International Education Project (not for profit Foundation) during the conduct of the study, with personal fees from BMS, Gilead and ViiV outside the submitted work. F. J. P. reports personal fees from Gilead Sciences, Janssen Pharmaceuticals, Merck, and BMS outside the submitted work. G. P. reports personal fees from Gilead, Abbvie, BMS, ViiV, GSK, Janseen, nephrotek outside the submitted work. J. E. L. reports personal fees from Merck outside the submitted work. T. T. B. reports personal fees from EMD-Serono, Abbvie, Gilead, ViiV grants and personal fees from Merck and grants from GSK outside the submitted work. The remaining authors have none to declare.
Acknowledgements
We thank the dedicated MACS participants. Data from this study were previously presented, in part, at the 15th International Workshop on Adverse Drug Reactions and Co-Morbidities in HIV, Bruxelles, 2013 (oral presentation) and at the 21st Conference on Retrovirus and Opportunistic Infections, Boston, MA, 2014 (poster 63).
Multicenter AIDS Cohort Study members
The Multicenter AIDS Cohort Study (MACS) includes the following: Baltimore: The Johns Hopkins University Bloomberg School of Public Health: Joseph B. Margolick (PI), Barbara Crain, Adrian Dobs, Homayoon Farzadegan, Joel Gallant, Lisette Johnson-Hill, Michael Plankey, Ned Sacktor, Ola Selnes, James Shepard, Chloe Thio. Chicago: Feinberg School of Medicine, Northwestern University and Cook County Bureau of Health Services: Steven M. Wolinsky (PI), John P. Phair, Sheila Badri, Maurice O'Gorman, David Ostrow, Frank Palella, Ann Ragin. Los Angeles: University of California, UCLA Schools of Public Health and Medicine: Roger Detels (PI), Otoniel Martínez-Maza (Co-P I), Aaron Aronow, Robert Bolan, Elizabeth Breen, Anthony Butch, Beth Jamieson, Eric N. Miller, John Oishi, Harry Vinters, Dorothy Wiley, Mallory Witt, Otto Yang, Stephen Young, Zuo Feng Zhang. Pittsburgh: University of Pittsburgh, Graduate School of Public Health: Charles R. Rinaldo (PI), Lawrence A. Kingsley (Co-PI), James T. Becker, Ross D. Cranston, Jeremy J. Martinson, John W. Mellors, Anthony J. Silvestre, Ronald D. Stall. Data Coordinating Center: The Johns Hopkins University Bloomberg School of Public Health: Lisa P. Jacobson (PI), Alvaro Munoz (Co-PI), Alison Abraham, Keri Althoff, Christopher Cox, Gypsyamber D'Souza, Elizabeth Golub, Janet Schollenberger, Eric C. Seaberg, Sol Su. NIH: National Institute of Allergy and Infectious Diseases: Robin E. Huebner; National Cancer Institute: Geraldina Dominguez. Web site located at http://www.statepi.jhsph.edu/macs/macs.html.
References
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Detels R, Munoz A, McFarlane G, et al. Effectiveness of potent antiretroviral therapy on time to AIDS and death in men with known HIV infection duration. Multicenter AIDS Cohort Study Investigators. JAMA. 1998;280:1497–503. doi: 10.1001/jama.280.17.1497. [DOI] [PubMed] [Google Scholar]
- 3.Hessol NA, Kalinowski A, Benning L, et al. Mortality among participants in the Multicenter AIDS Cohort Study and the Women's Interagency HIV Study. Clin Infect Dis. 2007;44:287–94. doi: 10.1086/510488. [DOI] [PubMed] [Google Scholar]
- 4.Brown TT, Cole SR, Li X, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Int Med. 2005;165:1179–84. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- 5.Brown TT, Li X, Cole SR, et al. Cumulative exposure to nucleoside analogue reverse transcriptase inhibitors is associated with insulin resistance markers in the Multicenter AIDS Cohort Study. AIDS. 2005;19:1375–83. doi: 10.1097/01.aids.0000181011.62385.91. [DOI] [PubMed] [Google Scholar]
- 6.Tien PC, Schneider MF, Cole SR, et al. Antiretroviral therapy exposure and incidence of diabetes mellitus in the Women's Interagency HIV Study. AIDS. 2007;21:1739–45. doi: 10.1097/QAD.0b013e32827038d0. [DOI] [PubMed] [Google Scholar]
- 7.Capeau J, Bouteloup V, Katlama C, et al. Ten-year diabetes incidence in 1046 HIV-infected patients started on a combination antiretroviral treatment. AIDS. 2012;26:303–14. doi: 10.1097/QAD.0b013e32834e8776. [DOI] [PubMed] [Google Scholar]
- 8.De Wit S, Sabin CA, Weber R, et al. Incidence and risk factors for new-onset diabetes in HIV-infected patients: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study. Diabetes Care. 2008;31:1224–9. doi: 10.2337/dc07-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–12. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.International Expert C. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–34. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diop ME, Bastard JP, Meunier N, et al. Inappropriately low glycated hemoglobin values and hemolysis in HIV-infected patients. AIDS Res Hum Retroviruses. 2006;22:1242–7. doi: 10.1089/aid.2006.22.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim PS, Woods C, Georgoff P, et al. A1C underestimates glycemia in HIV infection. Diabetes Care. 2009;32:1591–3. doi: 10.2337/dc09-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glesby MJ, Hoover DR, Shi Q, et al. Glycated haemoglobin in diabetic women with and without HIV infection: data from the Women's Interagency HIV Study. Antivir Ther. 2010;15:571–7. doi: 10.3851/IMP1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaslow RA, Ostrow DG, Detels R, et al. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126:310–8. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 15.Koga M, Morita S, Saito H, et al. Association of erythrocyte indices with glycated haemoglobin in pre-menopausal women. Diabet Med. 2007;24:843–7. doi: 10.1111/j.1464-5491.2007.02161.x. [DOI] [PubMed] [Google Scholar]
- 16.Schenker EL, Hultin LE, Bauer KD, et al. Evaluation of a dual-color flow cytometry immunophenotyping panel in a multicenter quality assurance program. Cytometry. 1993;14:307–17. doi: 10.1002/cyto.990140311. [DOI] [PubMed] [Google Scholar]
- 17.1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 18.Adler AI, Shaw EJ, Stokes T, et al. Newer agents for blood glucose control in type 2 diabetes: summary of NICE guidance. BMJ. 2009;338:b1668. doi: 10.1136/bmj.b1668. [DOI] [PubMed] [Google Scholar]
- 19.Polgreen PM, Putz D, Stapleton JT. Inaccurate glycosylated hemoglobin A1C measurements in human immunodeficiency virus-positive patients with diabetes mellitus. Clin Infect Dis. 2003;37:e53–6. doi: 10.1086/376633. [DOI] [PubMed] [Google Scholar]
- 20.Marchand A, Galen RS, Van Lente F. The predictive value of serum haptoglobin in hemolytic disease. JAMA. 1980;243:1909–11. [PubMed] [Google Scholar]
- 21.Steele RH, Keogh GL, Quin J, et al. Mean cell volume (MCV) changes in HIV-positive patients taking nucleoside reverse transcriptase inhibitors (NRTIs): a surrogate marker for adherence. Int J STD AIDS. 2002;13:748–54. doi: 10.1258/095646202320753691. [DOI] [PubMed] [Google Scholar]
- 22.Romanelli F, Empey K, Pomeroy C. Macrocytosis as an indicator of medication (zidovudine) adherence in patients with HIV infection. AIDS Patient Care STDs. 2002;16:405–11. doi: 10.1089/108729102760330245. [DOI] [PubMed] [Google Scholar]
- 23.Geene D, Sudre P, Anwar D, et al. Causes of macrocytosis in HIV-infected patients not treated with zidovudine. Swiss HIV Cohort Study. J Infect. 2000;40:160–3. doi: 10.1053/jinf.1999.0628. [DOI] [PubMed] [Google Scholar]
- 24.Moldawer LL, Marano MA, Wei H, et al. Cachectin/tumor necrosis factor-alpha alters red blood cell kinetics and induces anemia in vivo. FASEB J. 1989;3:1637–43. doi: 10.1096/fasebj.3.5.2784116. [DOI] [PubMed] [Google Scholar]
- 25.Schaer DJ, Schleiffenbaum B, Kurrer M, et al. Soluble hemoglobin-haptoglobin scavenger receptor CD163 as a lineage-specific marker in the reactive hemophagocytic syndrome. Eur J Haematol. 2005;74:6–10. doi: 10.1111/j.1600-0609.2004.00318.x. [DOI] [PubMed] [Google Scholar]
- 26.Davidson MB, Schriger DL. Effect of age and race/ethnicity on HbA1c levels in people without known diabetes mellitus: implications for the diagnosis of diabetes. Diabetes Res Clin Pract. 2010;87:415–21. doi: 10.1016/j.diabres.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 27.Nakashima K, Nishizaki O, Andoh Y. Acceleration of hemoglobin glycation with aging. Clin Chim Acta. 1993;215:111–8. doi: 10.1016/0009-8981(93)90254-2. [DOI] [PubMed] [Google Scholar]
- 28.Herman WH, Cohen RM. Racial and ethnic differences in the relationship between HbA1c and blood glucose: implications for the diagnosis of diabetes. J Clin Endocrinol Metab. 2012;97:1067–72. doi: 10.1210/jc.2011-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selvin E, Steffes MW, Ballantyne CM, et al. Racial differences in glycemic markers: a cross-sectional analysis of community-based data. Ann Intern Med. 2011;154:303–9. doi: 10.1059/0003-4819-154-5-201103010-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nathan DM, Kuenen J, Borg R, et al. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31:1473–8. doi: 10.2337/dc08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]