Abstract
Objectives
Over-the-counter access to an inexpensive, effective topical microbicide could reduce the transmission of HIV and would increase women's control over their health and eliminate the need to obtain their partners' consent for prophylaxis. Chronic infection with herpes simplex virus 2 (HSV-2), also known as human herpes virus 2, has been shown to facilitate HIV infection and speed the progression to immunodeficiency disease. Our objective is to develop a drug formulation that protects against both HSV-2 and HIV infection and adheres to the vaginal surface with extended residence time.
Methods
We developed a formulation using two approved antiviral active pharmaceutical ingredients, aciclovir and tenofovir, in a novel bioadhesive vaginal delivery platform (designated SR-2P) composed of two polymers, poloxamer 407 NF (Pluronic® F-127) and polycarbophil USP (Noveon® AA-1). The efficacy of the formulation to protect from HSV-2 infection was tested in vitro and in vivo. In addition to its efficacy, it is essential for a successful microbicide to be non-irritating to the vaginal mucosa. We therefore tested our SR-2P platform gel in the FDA gold-standard microbicide safety model in rabbits and also in a rat vaginal irritation model.
Results
Our studies indicated that SR-2P containing 1% aciclovir and 5% tenofovir protects (i) Vero cells from HSV-2 infection in vitro and (ii) mice from HSV-2 infection in vivo. Our results further demonstrated that SR-2P was not irritating in either vaginal irritation model.
Conclusions
We conclude that SR-2P containing aciclovir and tenofovir may be a suitable candidate microbicide to protect humans from vaginal HSV-2 infection.
Keywords: HSV-2, microbicide, antiviral, vaginal, acyclovir
Introduction
Over-the-counter access to an inexpensive, effective topical microbicide could reduce the transmission of HIV and would increase women's control over their health and eliminate the need to obtain their partners' consent for prophylaxis. Microbicides with different mechanisms have been developed and tested in clinical trials.1 The effectiveness of most microbicide technologies tested to date has been discouraging. First-generation surfactants such as nonoxynol-9 irritated vaginal tissues and increased susceptibility to infection.2 Subsequent trials tested polymers generally regarded as safe, which were vaginal defence enhancers or mucosal pathogen entry blockers. Even though clinical trials demonstrated that they are safe to use, they did not offer statistically improved protection from HIV infection despite promising pre-clinical antiviral results.3 Using the viral replication blocker tenofovir4 formulated in a hydroxyethylcellulose (HEC) gel, the CAPRISA 004 trial is considered a breakthrough in microbicide development since it demonstrated a statistically significant reduction in HIV incidence by up to 54%.5 This achieved protection from HIV infection observed in the CAPRISA 004 trial is encouraging but highlights the need for further improvement.
Chronic infection with herpes simplex virus 2 (HSV-2), also known as human herpes virus 2, has been shown to facilitate HIV infection and speed the progression to immunodeficiency disease.6,7 This correlation has been particularly noted in Africa8 and among African Americans,9 indicating the importance of developing a microbicide that inhibits infection from both HIV and HSV. HSV-2 infection is a global epidemic, with up to 80% prevalence in general female populations in Africa.10 Our objective is therefore to develop a drug formulation that protects against both HIV and HSV infection using two approved antiviral active pharmaceutical ingredients (APIs): the guanosine analogue aciclovir11 and the nucleotide analogue reverse transcriptase inhibitor tenofovir.12 The combination of these drugs has been tested by others in vaginal delivery platforms such as intravaginal rings.13,14 Even though the findings from these studies are promising, it is necessary to provide choices between different delivery platforms to enable women to choose based on their personal preference, social environment and environmental conditions.15 Our objective is therefore to deliver these APIs, aciclovir and tenofovir, using a bioadhesive vaginal delivery platform16 composed of two FDA-approved polymers, poloxamer 407 NF (Pluronic® F-127) and polycarbophil USP (Noveon® AA-1). The antiviral properties of this combination drug formulation against HSV-2 infection are described here, whereas the protection from HIV infection is subject to separate studies. Poloxamer 407 NF is temperature sensitive whereas polycarbophil USP is mucoadhesive. These polymers are prepared in separate compartments and are mixed just before administration to the vagina. Upon mixing of the two polymers and administration to the vagina, poloxamer 407 NF and polycarbophil USP undergo phase transition from solution to gel due to a temperature rise from ambient to 37°C and pH increase.17 We believe that the combination gel forms micelle-like particles that adhere to the vaginal wall and releases aciclovir and tenofovir over prolonged periods of time because of its extended residence time. Gel formulations containing different concentrations of these two polymers were tested for their characteristics such as pH, osmolality, buffering capacity, viscosity and bioadhesiveness.17 Based on these studies, the optimized vaginal platform gel composition, SR-2P, was selected. The goal of our studies described here is to test the antiviral potency of SR-2P containing aciclovir and tenofovir to protect from HSV-2 infection in vitro and in vivo and to test its safety in the FDA gold-standard rabbit vaginal irritation model18 as well as a complementary rat vaginal irritation model.
Materials and methods
Chemicals
Depot medroxyprogesterone (Depo Provera™) was obtained from the local pharmacy (Leiter's, San Jose, CA, USA). HEC, carboxymethylcellulose (CMC) and benzalkonium chloride (BZK) were obtained from Sigma (St Louis, MO, USA). Tenofovir was obtained from Hangzhou Starshine Pharmaceutical (Shanghai, China). Polycarbophil USP was received as a gift sample from Lubrizol (Wickliffe, OH, USA). Poloxamer 407 NF, aciclovir, glacial acetic acid, lactic acid, BSA, zinc chloride, magnesium chloride, calcium hydroxide, monosodium dihydrogen phosphate, sodium hydroxide, disodium monohydrogen phosphate, potassium hydroxide, glucose, fructose, urea, lactic acid, potassium chloride, methylparaben, propylparaben, trisodium citrate and N-9 were purchased from Spectrum Chemicals Manufacturing Company (Gardena, CA, USA). Vaginal fluid simulant (VFS) and seminal fluid simulant (SFS) were prepared as previously described.19,20
Test article preparation
Test articles SR-2P, SR-2P containing 5% aciclovir and 1% tenofovir (SR-2P/A + T), each with and without preservatives were prepared as described.17 Briefly, gels were prepared in double-barrel syringes containing aqueous gel solutions of poloxamer 407 NF (1.0% w/w) and polycarbophil USP (1.0% w/w) in individual compartments. Tenofovir was included at 2% (w/w) in the poloxamer 407 NF compartment and aciclovir was included at 10% (w/w) in the polycarbophil USP compartment. Samples containing preservatives were prepared by including methylparaben and propylparaben at 0.18% and 0.02% (w/w), respectively, to each of the two compartments. The bioadhesive combination formulations were prepared immediately before use by mixing the contents of the two compartments and extruding the combination as a gel. As reference control articles, 2.7% (w/v) HEC gel was prepared in aqueous solution and 5% (w/v) aciclovir was suspended and sonicated in 2.7% (w/v) HEC gel (HEC/aciclovir).
Cells and viruses
African green monkey kidney epithelial (Vero) cells were originally obtained from ATCC (Manassas, MD, USA). Vero cells were propagated in Vero medium [RPMI-1640 containing 5% newborn calf serum (NBCS) and additional 4 mM l-glutamine]. HSV-2 G strain was originally obtained from ATCC (VR-734). HSV-2 was propagated in Vero cells similarly as previously described.21 Vero cells were seeded at ∼2.5 × 104 cells/cm2. The next day, medium was removed and cells were washed with assay buffer (AB; Hanks balanced salt solution with Ca2+/Mg2+, 4 mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin and 25 mM HEPES). Cells were infected with HSV-2 in AB. After 2 h, the virus-containing supernatant was replaced with Vero medium. After 2–3 days, when the cytopathic effects of HSV-2 infection became most prominent, cells were disrupted by freezing at −60°C or below. Virus-containing cell lysate–supernatant suspensions were collected, sonicated for 5 min and stored in single-use aliquots at −60°C or below.
Animals
BALB/c mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). New Zealand White (NZW) rabbits were obtained from Charles River Laboratories (Wilmington, MA, USA). Sprague Dawley rats were obtained from Harlan Laboratories (Indianapolis, IN, USA). All animal studies were approved by the Veterans Affairs' or SRI International's Institutional Animal Care and Use Committees in full compliance with all regulations of the National Institutes of Health Office of Laboratory Animal Welfare.
Viral infection assays
Virus stocks and microbrush samples were tested in cytoprotection assays. Vero cells were seeded at 5 × 103/well in black clear-bottomed 96-well microtitre plates (Greiner Bio-One, Monroe, NC, USA). The following day, supernatant was removed, cells were washed with AB and 0.030 mL of AB was added to each well. Serial dilutions of the virus stocks were prepared in AB and 0.030 mL of diluted virus or microbrush samples was added in duplicate. After 1 h, virus infection was stopped by addition of 0.030 mL of 15% NBCS medium (RPMI-1640, 15% NBCS, 4 mM glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin and 25 mM HEPES) supplemented with 10 μg/mL human immunoglobulin (Sigma). This blocking step utilizes the fact that HSV encodes a receptor that binds the Fc portion of human immunoglobulin.21 After 2–4 days, cell proliferation was measured by the CellTiter-Glo® assay according to the manufacturer's instructions (Promega, Madison, WI, USA). Luminescence was read using a microplate reader (SpectraMAX M5; Molecular Devices, Sunnyvale, CA, USA). Luminescence data were normalized to wells containing the highest virus concentration as 100% infectivity and no virus as 0% infectivity. Tissue culture ID50 (TCID50) values were determined by non-linear regression.
Antiviral cytoprotection assays
Antiviral activity of test articles was measured in HSV-2 cytoprotection assays. Vero cells were seeded as described for the viral infection assays above. Supernatant was removed and cells were washed with AB. Dilutions of test articles (SR-2P, SR-2P/A + T, each with and without preservatives) or control articles were prepared in AB and 0.030 mL of sample was added to individual wells in duplicate. Virus stocks were diluted to 50× TCID50 and 0.030 mL was added. Virus infection was blocked after 1 h by addition of 0.030 mL of 15% NBCS medium supplemented with 10 μg/mL human immunoglobulin. After 2–4 days, cell proliferation was measured as described above. Luminescence data were normalized to negative control wells as 100% infectivity and input wells (measured when cells were infected) as 0% infectivity. Effective doses (ED)50 were determined by non-linear regression.
Cytotoxicity assays
The cytotoxicity of test articles was measured in viability assays. Vero cells were seeded as described for the viral infection assays above. Supernatant was removed and cells were washed with AB. Dilutions of test or control articles were prepared in AB and 0.030 mL of sample was added to individual wells in duplicate. Subsequently, 0.030 mL of AB and 0.030 mL of 15% NBCS medium supplemented with 10 μg/mL human immunoglobulin were added. After 2–4 days, cell proliferation was measured as described above. Luminescence data were normalized to wells containing the highest test article dilution as 100% viability and negative control wells as 0% viability.
Antiviral HSV-2 in vivo studies
Female 6–10-week-old BALB/c mice were conditioned with 3 mg depot medroxyprogesterone by subcutaneous injection twice within 1week before study initiation. The vaginal vault of the mice was rinsed with 0.050 mL of saline intravaginally using a 20 gauge blunt applicator (Microbrush, Grafton, WI, USA). Mice were treated with 0.020 mL of test or control articles intravaginally on day 0. After 1 h, mice were inoculated intravaginally with 0.030 mL of HSV-2 G strain (TCID50/mL = 5.2 × 104). Mice were monitored daily for clinical signs of infection such as swelling, redness, hair loss or lesions in the vaginal area and/or paralysis. Mice that showed these signs were considered infected, euthanized and assumed dead at the following observation timepoint. On days 4 and 6, microbrush samples were collected from the vaginal vault, transferred to collection tubes containing 0.2 mL of AB and stored at −60°C or below for viral infection assays. Vaginal tissues were collected at euthanasia and fixed in neutral-buffered formalin for ≥24 h for histopathology analysis.
Rabbit vaginal irritation in vivo studies
Vaginal irritation was measured essentially as described previously.18,22 Briefly, female nulliparous 18–22-week-old NZW rabbits were dosed for 10 consecutive days intravaginally using a steel ball-tipped cannula with 1 mL of test or control articles. One day after the last test article administration, animals were euthanized. The vagina was excised and formalin-fixed for histopathology evaluation.
Rat vaginal irritation in vivo studies
Vaginal irritation was measured in female 6–10-week-old Sprague Dawley rats (Harlan, Indianapolis, IN, USA) similarly to the rabbit vaginal irritation model. Animals were dosed intravaginally with 0.050 mL of test or control articles once daily for 10 days using a 20 gauge blunt applicator inserted 0.8 cm into the vaginal vault. On day 11, animals were euthanized; vaginal tissues were excised and formalin-fixed for histopathology analysis.
Histopathology
Histopathology was analysed semi-quantitatively as previously described.22 Briefly, microtome sections were prepared from paraffin-embedded formalin-fixed tissues, stained with haematoxylin and eosin and scored by a board-certified pathologist. Microscopic changes were coded by the most specific topographical and morphological diagnosis. Systematized Nomenclature of Medicine and National Toxicology Program terminology manuals were used as guidelines. Gradable observations (congestion, epithelial thinning, epithelial vacuolation, erosion, exudate, haemorrhage, keratinization, leucocyte infiltration, mucosal necrosis and mucosal single-cell vacuolation) were scored semi-quantitatively by a four-step grading system: 0, not observed; 1, minimal; 2, mild; 3, moderate; and 4, marked.
Statistical analysis
Statistical analysis was performed using Prism (GraphPad, La Jolla, CA, USA). Survival time was analysed by one-way analysis of variance (ANOVA) and Dunnett's test. Histopathology data were analysed by the Kruskal–Wallis test. P values <0.05 were considered statistically significant.
Results
HSV-2 cytoprotection in vitro studies
The antiviral activity of SR-2P containing 5% aciclovir and 1% tenofovir (SR-2P/A + T) was tested in cytoprotection assays in vitro with HSV-2 G strain. Concentrations of aciclovir and tenofovir are based on currently FDA-approved (Zovirax®) or clinically tested drugs.5,23 Cells were treated with multiple dilutions of SR-2P/A + T or 5% aciclovir in DMSO (reference control), followed by inoculation with HSV-2 G strain. Viral infectivity was blocked by addition of human immunoglobulin to study the prophylactic effect of the test articles to protect from HSV-2 infection. Cell proliferation was measured by metabolic assay 4 days after viral infection. Our studies indicate that SR-2P/A + T and 5% aciclovir in DMSO limit the viral infectivity similarly (Figure 1a). However, at dilutions <103 the drugs are cytotoxic in vitro (Figure 1b), which may limit the maximum inhibition of viral infectivity observed in the viral infectivity assays (Figure 1a). Because of this cytotoxicity, we excluded drug dilutions of <103 from our studies and ED50 was calculated from drug dilutions ≥103, which may have negatively impacted confidence intervals. The apparent ED50 of SR-2P/A + T is 6.1 × 104 dilutions (concentration of aciclovir 0.8 μg/mL), similar to the ED50 of the aciclovir reference control [4.3 × 104 (concentration of aciclovir 1.2 μg/mL)]. SR-2P/A + T protects Vero cells from infection with HSV-2.
Figure 1.
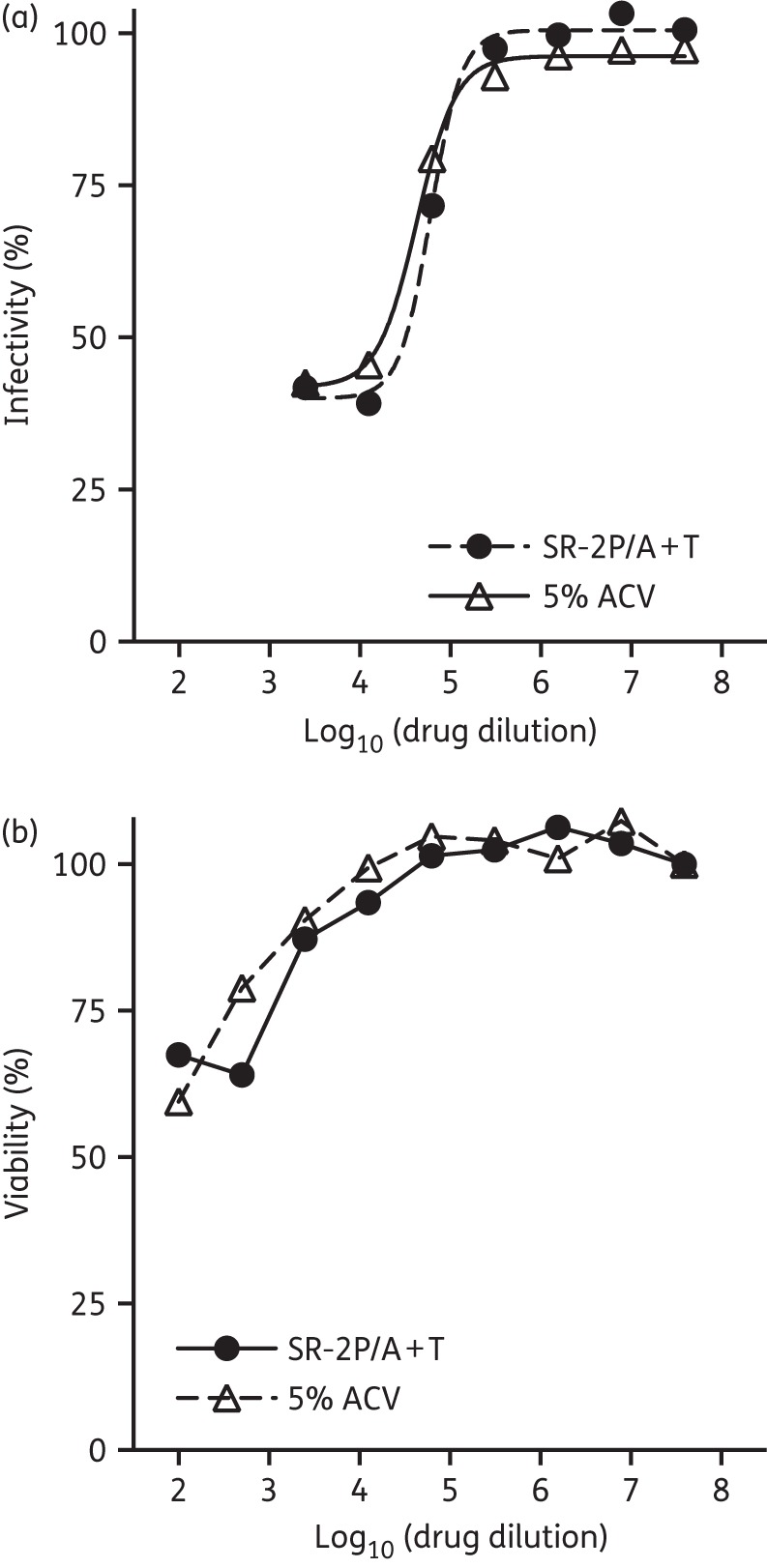
SR-2P containing 5% aciclovir and 1% tenofovir (SR-2P/A + T) protected Vero cells from HSV-2 infection similarly to 5% aciclovir in DMSO (5% ACV). Vero cells were treated with SR-2P/A + T (filled circles) or 5% ACV (open triangles) as a reference control, followed by infection with HSV-2 G strain for 1 h until viral infection was blocked by addition of human immunoglobulin. Cell proliferation was measured after 4 days and infectivity was calculated (a). Cytotoxicity of the test articles was measured after 4 days (b). SR-2P/A + T and 5% ACV were similarly cytotoxic at low dilutions, with no or limited cytotoxicity at concentrations ≥103. At dilutions >103, both SR-2P/A + T and 5% ACV similarly protected Vero cells from HSV-2 infection.
Because a marketed drug would typically have to include preservatives in its final formulation, we investigated whether the addition of preservatives would affect the antiviral efficacy or cytotoxicity of SR-2P/A + T. To address this question, we compared the cytoprotection of drug formulation with and without preservatives. The addition of methylparaben [0.18% (w/w)] and propylparaben [0.02% (w/w)] to either SR-2P without any API or SR-2P/A + T did not affect their cytoprotection against HSV-2 infection (Figure 2a) or their cytotoxicity on Vero cells (Figure 2b). We also asked whether the efficacy of our drugs is limited by the presence of vaginal or seminal fluids. We incubated SR-2P/A + T in VFS or SFS for 1 h before the cytoprotection assay. No significant loss of cytoprotection was observed when SR-2P/A + T was exposed to VFS or SFS (Figure 2c).
Figure 2.
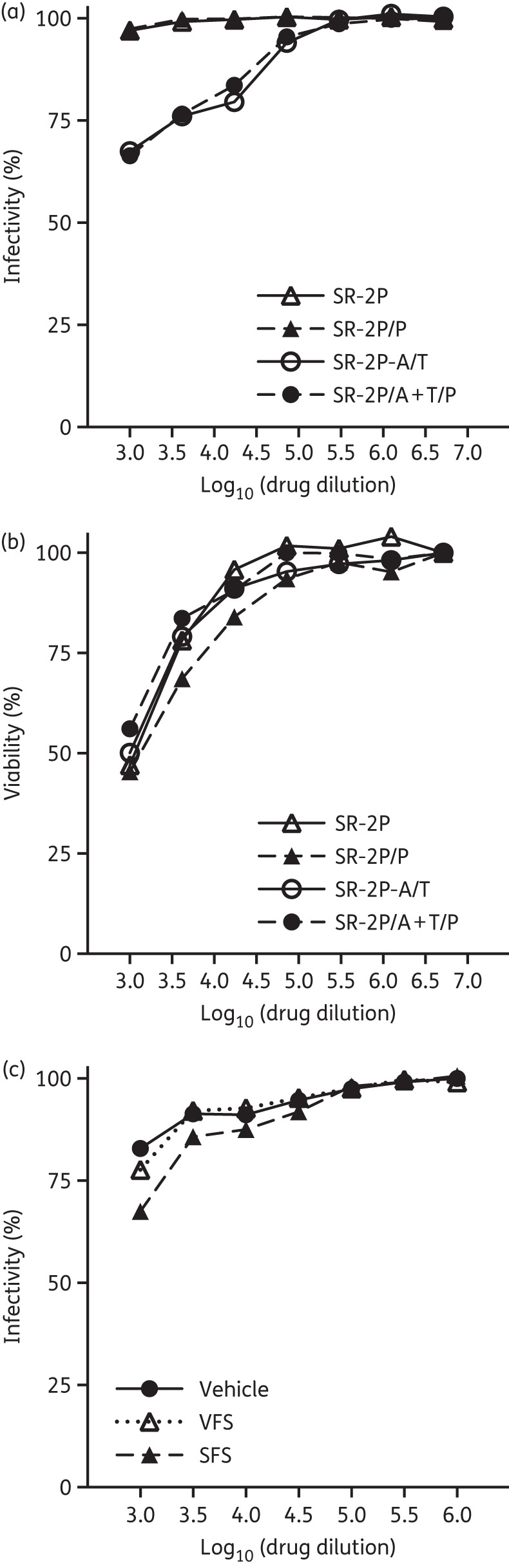
SR-2P containing 5% aciclovir and 1% tenofovir (SR-2P/A + T) protects similarly from viral infectivity with and without preservatives and is not affected by vaginal fluid simulants (VFS) and seminal fluid simulants (SFS). Vero cells were treated with preservative-free SR-2P (open triangles) and SR-2P/A + T (open circles) as well as preservative-containing [0.18% (w/w) methylparaben and 0.02% (w/w) propylparaben] SR-2P/P (filled triangles) and SR-2P/A + T/P (filled circles) at indicated dilutions followed by inoculation with HSV-2 G strain. Cell proliferation was measured after 4 days and viral infectivity was calculated (a). Viability was measured in the absence of virus after 4 days and cytotoxicity was calculated (b). SR-2P/A + T containing preservatives was diluted 1 : 10 in VFS (open triangles), SFS (filled triangles) or vehicle control (filled circles) and incubated for 1 h at 37°C before addition to the Vero cells at indicated dilutions. Cells were infected with HSV-2, viability was measured after 4 days and infectivity was calculated (c).
HSV-2 mouse in vivo studies
Because our gel is formulated for improved adhesion at increased vaginal temperature, we next tested its efficacy to protect from HSV-2 infection in vivo. Progesterone-conditioned mice were sham-treated or treated intravaginally with 2.7% HEC, SR-2P, SR-2P/A + T (n = 6) or 5% aciclovir in 2.7% HEC (HEC/aciclovir; n = 4). We did not observe leakage of the test articles until the animals were inoculated intravaginally with HSV-2 G strain (TCID50/mL = 5.2 × 104) 1 h after test article administration. We observed significantly (P < 0.05) increased survival in animals treated with SR-2P/A + T when compared with HEC-treated virus-infected animals (Figure 3). The median survival of the HEC vehicle-treated group was 8 days, which was unchanged after treatment with SR-2P. In contrast, SR-2P/A + T treatment increased the median survival to 13.5 days, beyond the 10.5 days median survival that was observed in animals treated with the HEC/aciclovir reference formulation (Figure 3).
Figure 3.
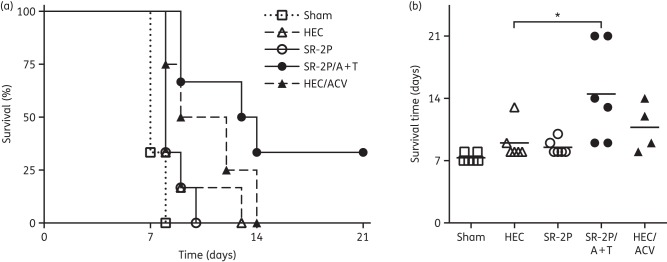
SR-2P containing 5% aciclovir and 1% tenofovir (SR-2P/A + T) significantly increases the survival of mice inoculated with HSV-2. Mice were sham-treated (open squares) or treated intravaginally with 2.7% hydroxyethylcellulose (HEC; open triangles), SR-2P without APIs (open circles), SR-2P/A + T (filled circles) (n = 6) or 2.7% HEC containing 5% aciclovir (HEC/ACV; filled triangles; n = 4). Subsequently, animals were inoculated intravaginally with HSV-2. Mice were monitored daily for clinical signs of infection. Mice that showed clinical signs of infection were euthanized. Two mice in the SR-2P/A + T-treated group survived until day 21. A Kaplan–Meier survival curve is shown in (a) and survival time is shown in (b). Data were statistically analysed by ANOVA and Dunnett's test in comparison to the HEC-treated study group. *P < 0.05.
Vaginal brush samples were collected on days 4 and 6 after viral inoculation to measure asymptomatic viral shedding, which may occur in the absence of clinical signs of HSV-2 infection. Samples were tested in viral infection studies and TCID50 were calculated based on a virus standard curve. Our experiments suggest increased TCID50 in vaginal brush samples from virus-inoculated vehicle-treated animals and decreased TCID50 in vaginal brush samples from virus-inoculated SR-2P/A + T-treated animals; however, none of these findings was statistically significant (data not shown).
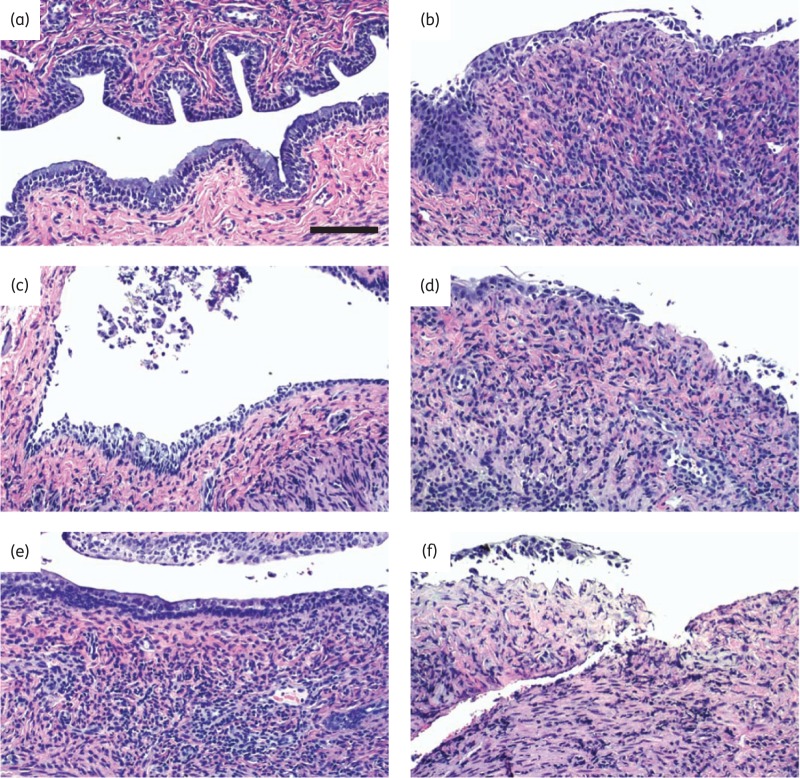
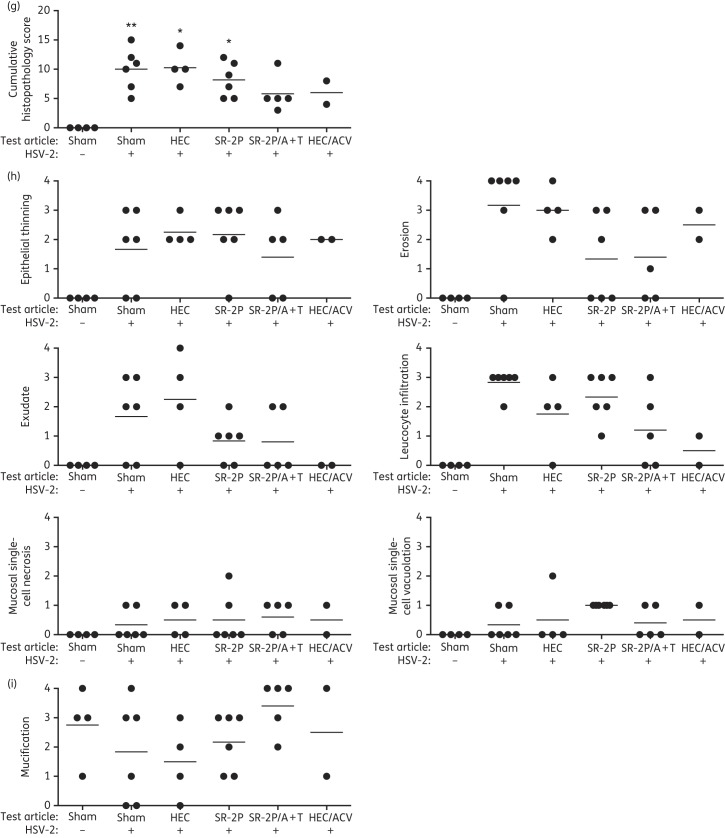
Vaginal tissues were collected during interim necropsies or at the conclusion of the study. Two sham-treated non-infected animals were euthanized at day 13 and at conclusion of the study. Formalin-fixed tissues were processed for histopathology evaluation by a board-certified pathologist in a blinded fashion. Five animals were excluded from the analysis because the animals had been found dead before interim necropsies could be performed (one animal each in the HEC-, SR-2P/A + T- and HEC/aciclovir-treated groups) or tissue slides were inadequate for analysis (one animal each in the HEC- and HEC/aciclovir-treated groups). Pictures from representative animals from each treatment group [uninfected (a), or infected with HSV-2 after sham treatment (b), HEC vehicle treatment (c), SR-2P treatment (d), SR-2P/A + T treatment (e) or HEC/aciclovir reference control treatment (f) respectively] are shown in Figure 4(a–f). Cumulative histopathology (sum of epithelial thinning, erosion, exudate, leucocyte infiltration, mucosal single-cell necrosis and mucosal single-cell vacuolation) was increased with statistical significance after virus inoculation in sham- and vehicle-treated animals compared with non-infected control animals (Figure 4g). In contrast, no significant increase in histopathology was observed in SR-2P/A + T- and HEC/aciclovir-treated animals (Figure 4g). Individual histopathology parameters are shown in Figure 4(h). Mucification, which is observed in non-infected animals especially after oestrous synchronization with progesterone, is shown in Figure 4(i).
Figure 4.
SR-2P containing 5% aciclovir and 1% tenofovir (SR-2P/A + T) limits the histopathology following HSV-2 infection. Mice were sham-treated or treated intravaginally with 2.7% hydroxyethylcellulose (HEC), SR-2P without APIs, SR-2P/A + T (n = 6) or 2.7% HEC containing 5% aciclovir (HEC/ACV) (n = 4). Subsequently, animals were inoculated intravaginally with HSV-2. Uninfected mice were used as a control (n = 4). Vaginal tissues were collected during interim necropsies or at termination of the study and stained with haematoxylin and eosin. Histology slide pictures are shown for representative animals that were uninfected (a) or were virus-inoculated following sham (b), HEC (c), SR-2P (d), SR-2P/A + T (e) or HEC/ACV (f) treatment. Bar = 100 μm. Tissue slides from individual animals were analysed semi-quantitatively by a board-certified pathologist in a blinded fashion for histopathology parameters (epithelial thinning, erosion, exudate, leucocyte infiltration, mucosal single-cell necrosis and mucosal single-cell vacuolation), which were scored on a scale of 0–4: 0 = not observed; 1 = minimal; 2 = mild; 3 = moderate; and 4 = marked. Cumulative histopathology scores (g) summarize the individual observations (h). Mucification is shown in (i). Histopathology was significantly increased in sham- and vehicle-treated HSV-2-infected mice compared with uninfected control animals. No significant increase was observed in SR-2P/A + T- and HEC/ACV-treated animals. Data were analysed by Kruskal–Wallis test. *P < 0.05; **P < 0.01. Figure 4 appears in colour in the online version of JAC and in black and white in the print version of JAC.
Vaginal irritation safety studies
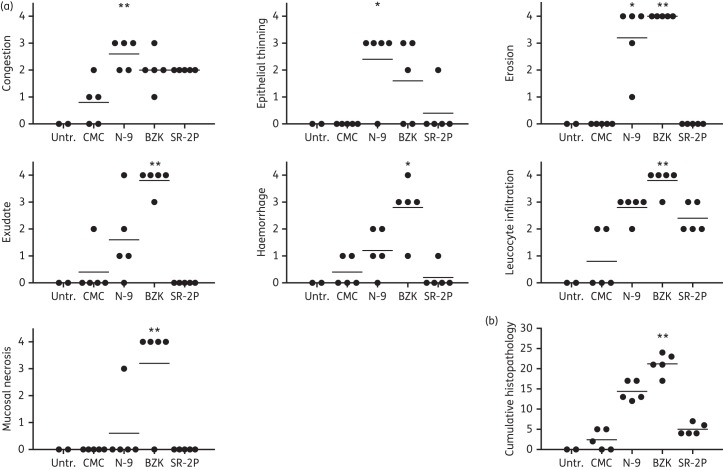
Not only does a successful microbicide have to be effective at blocking the viral infection, it also needs to be non-irritating to the vaginal mucosa. Vaginal irritants have been detrimental and increased viral infection in clinical trials.2 The FDA gold-standard model of vaginal safety is the rabbit vaginal irritation model. We therefore tested our SR-2P platform gel in this model. NZW rabbits were dosed once daily intravaginally for 10 days with SR-2P, with 2% CMC as non-irritating control as well as with 8% N-9 and 2% BZK as known vaginal irritants. An untreated control group was included in the study and histopathology was analysed semi-quantitatively by a board-certified pathologist. Whereas treatment with N-9 or BZK resulted in significantly increased histopathology observations such as congestion, epithelial thinning, erosion, exudate, haemorrhage, leucocyte infiltration or mucosal necrosis, no significant changes were observed after treatment with SR-2P (Figure 5a). When comparing cumulative histopathology, the observations in SR-2P-treated animals were comparable to those in the CMC vehicle control (Figure 5b).
Figure 5.
Semi-quantitative histopathology analysis in a rabbit vaginal irritation safety model. NZW rabbits were dosed intravaginally once daily with 2% carboxymethylcellulose (CMC; vehicle), 8% nonoxynol-9 (N-9) in 2% CMC, 2% benzalkonium chloride (BZK) or SR-2P (n = 5). Untreated animals (Untr.) were used as controls (n = 2). After 10 treatment days, vaginal tissues were collected and sections were stained with haematoxylin and eosin. Slides were analysed by a board-certified pathologist for individual histopathology parameters (congestion, epithelial thinning, erosion, exudate, haemorrhage, leucocyte infiltration and mucosal necrosis) and scored semi-quantitatively on a scale of 0–4: 0 = not observed; 1 = minimal; 2 = mild; 3 = moderate; and 4 = marked (filled circles). Cumulative histopathology scores were calculated as the sum of the individual parameters. Whereas N-9 and BZK treatment increased individual histopathology parameters significantly compared with CMC vehicle control treatment, no significant histopathology was observed after SR-2P treatment. Data were analysed by Kruskal–Wallis test. *P < 0.05; **P < 0.01.
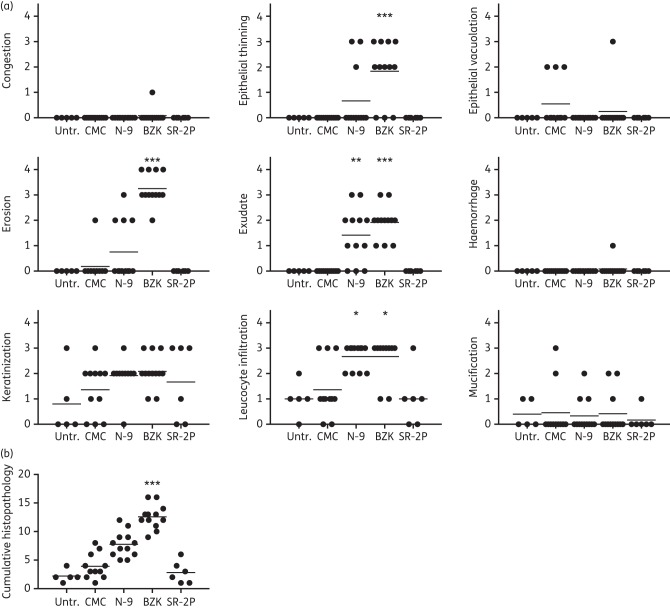
Vaginal irritation studies in rodents are a cost-effective way to evaluate the safety of candidate microbicides on multilayered vaginal epithelium in vivo, in contrast to studies in rabbits whose single-layer epithelium is very sensitive but also very different from the multilayered human vaginal epithelium. Sprague Dawley rats are a standard toxicology model and were dosed intravaginally with SR-2P, with 2% CMC as non-irritating control as well as with 8% N-9 and 2% BZK as known vaginal irritants. An untreated control group was included in the study and histopathology was analysed semi-quantitatively by a board-certified pathologist. Similar to what we observed in the rabbit vaginal irritation model, treatment with N-9 or BZK resulted in significantly increased histopathology observations such as epithelial thinning, erosion, exudate and leucocyte infiltration. In contrast, no significant changes were observed after treatment with SR-2P (Figure 6a). Cumulative histopathology in SR-2P-treated animals was comparable to the CMC vehicle control (Figure 6b).
Figure 6.
Semi-quantitative histopathology analysis in a rat vaginal irritation safety model. Sprague Dawley rats were dosed intravaginally once daily with 2% carboxymethylcellulose (CMC) vehicle (n = 11), 8% nonoxynol-9 (N-9) in 2% CMC (n = 12), 2% benzalkonium chloride (BZK; n = 12) or SR-2P (n = 6). Untreated animals (Untr.) were used as controls (n = 5). After 10 days, vaginal tissues were collected and sections were stained with haematoxylin and eosin. Slides were analysed by a board-certified pathologist for individual histopathology parameters (congestion, epithelial thinning, epithelial vacuolation, erosion, exudate, haemorrhage, keratinization, leucocyte infiltration and mucification) and scored semi-quantitatively on a scale of 0–4: 0 = not observed; 1 = minimal; 2 = mild; 3 = moderate; and 4 = marked. Cumulative histopathology scores were calculated as the sum of the individual parameters. Whereas 8% nonoxynol-9 and 2% BZK treatment increased individual histopathology parameters significantly compared with CMC vehicle control treatment, no significant histopathology was observed after SR-2P treatment. Data were analysed by Kruskal–Wallis test. *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
The goal of our studies is to develop and test a novel microbicide formulation that protects from HSV-2 and HIV-1 infection using a novel bioadhesive vaginal gel delivery platform,16,17 which delivers the APIs to the vaginal vault efficiently and with extended residence time, thereby increasing protection compared with current gel formulations. Here, we describe the efficacy of our formulation to protect from HSV-2 infection in vitro and in vivo and the safety of the formulation in two in vivo models of vaginal irritation.
We used Vero cells, which are a standard microbiology in vitro model for infectious disease pathogens including HSV-2.24 Leary et al.24 reported that aciclovir limits plaque formation in Vero cells following infection with wild-type HSV-2 strains SB5 and 333 at IC50s of 0.17–0.35 μg/mL, results that were similar to the apparent IC50 for aciclovir that we observed with HSV-2 G strain (1.2 μg/mL).
To limit microbial contamination of the final marketed drug, it may become necessary to add a preservative to the drug formulation; therefore, we included parabens in our formulation. Parabens are considered practically non-irritating and non-sensitizing in the population with normal skin.25 Importantly, parabens were used in the tenofovir gel that was successfully tested in the CAPRISA 004 trial.5,26 Our own results support these previous reports, which indicate that parabens are safe to use in vaginal microbicides. We did not observe increased cytotoxicity in our studies. Of equal importance, the antiviral in vitro efficacy of our drug formulation was not affected by the addition of parabens.
The cytoprotection of a vaginal gel may be affected by distinct conditions that can be observed in the vaginal milieu, such as the low pH vaginal environment or the presence of semen. These conditions can be simulated in vitro. The composition of vaginal and seminal fluids has been studied in great detail and based on these results VFS and SFS have been defined.19,20 Use of these simulants has been proposed and implemented in the evaluation of candidate microbicides.13,27–29 Our cytoprotection studies indicate that our formulation is stable both in VFS and SFS.
Our drug formulation is specifically designed for increased adhesion to the vaginal mucosa to protect locally from viral infection, conditions that can only be modelled in a rudimentary fashion in vitro. In vivo testing is therefore necessary to study the efficacy of our formulation. We tested SR-2P/A + T in a mouse vaginal HSV-2 infection model that has been used routinely by several research groups.30–35 These infection studies vary highly in their parameters such as age, strain and hormonal conditioning of the mice as well as viral strains, inoculation amounts and procedures. In our studies, we infected BALB/c mice with HSV-2 G strain. Mice had been conditioned with progesterone as described by us previously for mouse vaginal irritation studies.17 Progesterone conditioning increases the susceptibility of mice to HSV-2 infection.35 Our results therefore indicate that SR-2P/A + T protects highly susceptible mice from HSV-2 infection. Notably, in our study, the animals had been inoculated with HSV-2 1 h after test article administration. This suggests an extended protective window compared with previous reports by other research groups who inoculated mice within 20s30,31,36,37 or <10 min38,39 of test article administration. Previous time course studies suggest that the efficacy of microbicides typically decreases after ∼1 h.31,40,41 These differences in dosing and inoculation regimens may contribute to the different protective effects observed in HSV-2 infection studies in vivo.
We observed protection from vaginal HSV-2 infection in our in vivo HSV-2 infection study as indicated by reduced clinical signs of vaginal infection and histopathology. In this study, vaginal tissues were collected no earlier than 1 week after a single test article administration. Since no significant histopathology was observed following daily SR-2P treatments in a previous 12 day mouse vaginal irritation study,17 we believe that the histopathology observed in this vaginal infection study did not result from drug toxicity but were rather pathological signs of HSV-2 infection.
As an additional endpoint of this HSV-2 infection study, vaginal virus shedding was measured at two timepoints before clinical signs of infection occurred. Our experiments suggest reduced vaginal virus levels in SR-2P/A + T-treated virus-inoculated animals; however, these findings were not statistically significant. Studies reported by others used larger group sizes (n = 12–60) to achieve statistical significance in these types of studies.31,42 These group sizes suggest that vaginal virus levels are a less sensitive endpoint than clinical observations and histopathology. Clinical and histopathology may therefore be favourable endpoints, not only because they are standard pathology parameters, but also since this approach allows reduction of animal numbers in research. SR-2P/A + T provided statistically significant protection from HSV-2 infection in mice compared with vehicle-treated animals. Interestingly, SR-2P/A + T increased median survival beyond what we observed for our reference control HEC/aciclovir, which contains aciclovir in an HEC formulation resembling the gel formulation used in the CAPRISA 004 trial.5 These findings are particularly interesting because the specific bioadhesive properties of our platform gel are based on increased temperature.17 Since the clinical pathology in the mouse vaginal HSV-2 infection model differs from the human disease, the aim of future studies will be the efficacy of our formulation in alternate in vivo models such as the guinea pig HSV-2 infection model, which more closely resembles human genital herpes.43
The CAPRISA 004 trial demonstrated not only that 1% tenofovir protects women from HIV, but also from HSV-2 infection.5,44 Tenofovir offers less protection from HSV-2 infection when compared with its prodrug tenofovir disoproxil fumarate in vitro and in mouse vaginal infection studies in vivo.42,45 These findings suggest that tenofovir disoproxil fumarate would be favourable over tenofovir to develop a microbicide that protects from both HIV and HSV-2 infection. Unfortunately, tenofovir disoproxil fumarate undergoes hydrolysis in aqueous solutions and is therefore not suitable for aqueous gel formulations.46,47 The primary role of tenofovir in SR-2P/A + T is to protect from HIV infection, whereas the role of aciclovir is to protect from HSV-2 infection.
Vaginal microbicides not only have to effectively inhibit viral infection, but also need to be safe to use, especially when the drug is used repeatedly over extended periods of time. Past experience with N-9 has shown that candidate microbicides may increase the risk of viral infection in clinical trials2 even if the candidate drugs have appropriate antiviral properties.33 It is therefore extremely important that a candidate microbicide is non-irritating to the vaginal mucosa. Ultimately, a microbicide should be available over the counter without prescription, even though the initial drug product will be regulated by the FDA.48 Although the FDA has not provided specific guidance for microbicide development, they have outlined steps for its pre-clinical development.48–50 The FDA gold-standard for vaginal safety is the rabbit vaginal irritation model.18,49 Its advantages are the single-layer epithelium and the absence of an oestrous cycle, which makes the animals very sensitive and well suited to evaluate vaginal irritation by histopathology. Nevertheless, this model is far from ideal, since the vaginal histology and anatomy of rabbits and humans are very different. It would therefore be prudent to complement rabbit vaginal irritation studies with studies in rodents, which have a multilayered vaginal epithelium and are a cost-effective means of studying vaginal irritation in vivo with more statistical power. We previously reported the vaginal safety of SR-2P in a mouse vaginal irritation model.17 In this study, we report that SR-2P resulted in no vaginal toxicity when compared with nonoxynol-9 and BZK in rabbit and rat vaginal irritation models.
In summary, our studies suggest that SR-2P/A + T protects from HSV-2 infection both in vitro and in vivo. Our vaginal irritation studies further indicate that SR-2P is safe to use since it did not cause vaginal irritation. We conclude that SR-2P/A + T may be a suitable candidate microbicide to protect humans from vaginal HSV-2 infection.
Funding
This work was supported by Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services (grant number NIAID R21A1098658).
Transparency declarations
None to declare.
Disclaimer
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Acknowledgements
We thank SRI International's Toxicology Technical Services staff for their excellent assistance in performing the vaginal irritation studies. Additionally, we would like to thank Dr David Fairchild for his assistance in analysing the histopathology data.
References
- 1.Abdool Karim SS, Baxter C. Overview of microbicides for the prevention of human immunodeficiency virus. Best Pr Res Clin Obstet Gynaecol. 2012;26:427–39. doi: 10.1016/j.bpobgyn.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Damme L, Chandeying V, Ramjee G, et al. Safety of multiple daily applications of COL-1492, a nonoxynol-9 vaginal gel, among female sex workers. COL-1492 Phase II Study Group. AIDS. 2000;14:85–8. doi: 10.1097/00002030-200001070-00010. [DOI] [PubMed] [Google Scholar]
- 3.Obiero J, Mwethera PG, Hussey GD, et al. Vaginal microbicides for reducing the risk of sexual acquisition of HIV infection in women: systematic review and meta-analysis. BMC Infect Dis. 2012;12:289. doi: 10.1186/1471-2334-12-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piliero PJ. Pharmacokinetic properties of nucleoside/nucleotide reverse transcriptase inhibitors. J Acquir Immune Defic Syndr. 2004;37(Suppl 1):S2–12. doi: 10.1097/01.qai.0000137001.40505.56. [DOI] [PubMed] [Google Scholar]
- 5.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson SS, Fakioglu E, Herold BC. Novel approaches in fighting herpes simplex virus infections. Expert Rev Anti Infect Ther. 2009;7:559–68. doi: 10.1586/eri.09.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheth PM, Sunderji S, Shin LY, et al. Coinfection with herpes simplex virus type 2 is associated with reduced HIV-specific T cell responses and systemic immune activation. J Infect Dis. 2008;197:1394–401. doi: 10.1086/587697. [DOI] [PubMed] [Google Scholar]
- 8.Freeman EE, Orroth KK, White RG, et al. Proportion of new HIV infections attributable to herpes simplex 2 increases over time: simulations of the changing role of sexually transmitted infections in sub-Saharan African HIV epidemics. Sex Transm Infect. 2007;83(Suppl 1):i17–24. doi: 10.1136/sti.2006.023549. [DOI] [PubMed] [Google Scholar]
- 9.Ward H, Ronn M. Contribution of sexually transmitted infections to the sexual transmission of HIV. Curr Opin HIV AIDS. 2010;5:305–10. doi: 10.1097/COH.0b013e32833a8844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss H. Epidemiology of herpes simplex virus type 2 infection in the developing world. Herpes. 2004;11(Suppl 1):24A–35A. [PubMed] [Google Scholar]
- 11.Elion GB. Acyclovir: discovery, mechanism of action, and selectivity. J Med Virol. 1993;(Suppl 1):2–6. doi: 10.1002/jmv.1890410503. [DOI] [PubMed] [Google Scholar]
- 12.Pham PA, Gallant JE. Tenofovir disoproxil fumarate for the treatment of HIV infection. Expert Opin Drug Metab Toxicol. 2006;2:459–69. doi: 10.1517/17425255.2.3.459. [DOI] [PubMed] [Google Scholar]
- 13.Moss JA, Malone AM, Smith TJ, et al. Simultaneous delivery of tenofovir and acyclovir via an intravaginal ring. Antimicrob Agents Chemother. 2012;56:875–82. doi: 10.1128/AAC.05662-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baum MM, Butkyavichene I, Gilman J, et al. An intravaginal ring for the simultaneous delivery of multiple drugs. J Pharm Sci. 2012;101:2833–43. doi: 10.1002/jps.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rohan LC, Sassi AB. Vaginal drug delivery systems for HIV prevention. AAPS J. 2009;11:78–87. doi: 10.1208/s12248-009-9082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shankar GN, Burke RL. Bioadhesive delivery system for transmucosal delivery of beneficial agents. 2009 US Patent No. US 7592021 B2. [Google Scholar]
- 17.Podaralla S, Alt C, Shankar GN. Formulation development and evaluation of innovative two-polymer (SR-2P) bioadhesive vaginal gel. AAPS Pharm Sci Tech. 2014;15:928–38. doi: 10.1208/s12249-014-0124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eckstein P, Jackson MC, Millman N, et al. Comparison of vaginal tolerance tests of spermicidal preparations in rabbits and monkeys. J Reprod Fertil. 1969;20:85–93. doi: 10.1530/jrf.0.0200085. [DOI] [PubMed] [Google Scholar]
- 19.Owen DH, Katz DF. A vaginal fluid simulant. Contraception. 1999;59:91–5. doi: 10.1016/s0010-7824(99)00010-4. [DOI] [PubMed] [Google Scholar]
- 20.Owen DH, Katz DF. A review of the physical and chemical properties of human semen and the formulation of a semen simulant. J Androl. 2005;26:459–69. doi: 10.2164/jandrol.04104. [DOI] [PubMed] [Google Scholar]
- 21.Blaho JA, Morton ER, Yedowitz JC. Herpes simplex virus: propagation, quantification, and storage. Curr Protoc Microbiol. 2005 doi: 10.1002/9780471729259.mc14e01s00. Chapter 14: Unit 14E.1. [DOI] [PubMed] [Google Scholar]
- 22.Alt C, Harrison T, Dousman L, et al. Increased CCL2 expression and macrophage/monocyte migration during microbicide-induced vaginal irritation. Curr HIV Res. 2009;7:639–49. doi: 10.2174/157016209789973682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anon. ZOVIRAX® (acyclovir) cream 5% for topical use Initial U.S. Approval: 2002. 2014. http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021478s007lbl.pdf .
- 24.Leary JJ, Wittrock R, Sarisky RT, et al. Susceptibilities of herpes simplex viruses to penciclovir and acyclovir in eight cell lines. Antimicrob Agents Chemother. 2002;46:762–8. doi: 10.1128/AAC.46.3.762-768.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anon. Final amended report on the safety assessment of methylparaben, ethylparaben, propylparaben, isopropylparaben, butylparaben, isobutylparaben, and benzylparaben as used in cosmetic products. Int J Toxicol. 2008;27(Suppl 4):1–82. doi: 10.1080/10915810802548359. [DOI] [PubMed] [Google Scholar]
- 26.Nuttall J, Kashuba A, Wang R, et al. Pharmacokinetics of tenofovir following intravaginal and intrarectal administration of tenofovir gel to rhesus macaques. Antimicrob Agents Chemother. 2012;56:103–9. doi: 10.1128/AAC.00597-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lackman-Smith C, Osterling C, Luckenbaugh K, et al. Development of a comprehensive human immunodeficiency virus type 1 screening algorithm for discovery and preclinical testing of topical microbicides. Antimicrob Agents Chemother. 2008;52:1768–81. doi: 10.1128/AAC.01328-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li L, Qiao P, Yang J, et al. Maleic anhydride-modified chicken ovalbumin as an effective and inexpensive anti-HIV microbicide candidate for prevention of HIV sexual transmission. Retrovirology. 2010;7:37. doi: 10.1186/1742-4690-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tong P, Lu Z, Chen X, et al. An engineered HIV-1 gp41 trimeric coiled coil with increased stability and anti-HIV-1 activity: implication for developing anti-HIV microbicides. J Antimicrob Chemother. 2013;68:2533–44. doi: 10.1093/jac/dkt230. [DOI] [PubMed] [Google Scholar]
- 30.Zeitlin L, Whaley KJ, Hegarty TA, et al. Tests of vaginal microbicides in the mouse genital herpes model. Contraception. 1997;56:329–35. doi: 10.1016/s0010-7824(97)00154-6. [DOI] [PubMed] [Google Scholar]
- 31.Zeitlin L, Hoen TE, Achilles SL, et al. Tests of Buffergel for contraception and prevention of sexually transmitted diseases in animal models. Sex Transm Dis. 2001;28:417–23. doi: 10.1097/00007435-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Maguire RA, Bergman N, Phillips DM. Comparison of microbicides for efficacy in protecting mice against vaginal challenge with herpes simplex virus type 2, cytotoxicity, antibacterial properties, and sperm immobilization. Sex Transm Dis. 2001;28:259–65. doi: 10.1097/00007435-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Polsky B, Baron PA, Gold JW, et al. In vitro inactivation of HIV-1 by contraceptive sponge containing nonoxynol-9. Lancet. 1988;1:1456. doi: 10.1016/s0140-6736(88)92261-1. [DOI] [PubMed] [Google Scholar]
- 34.Krawczyk A, Krauss J, Eis-Hübinger AM, et al. Impact of valency of a glycoprotein B-specific monoclonal antibody on neutralization of herpes simplex virus. J Virol. 2011;85:1793–803. doi: 10.1128/JVI.01924-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parr MB, Kepple L, McDermott MR, et al. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab Invest. 1994;70:369–80. [PubMed] [Google Scholar]
- 36.Bourne N, Stegall R, Montano R, et al. Efficacy and toxicity of zinc salts as candidate topical microbicides against vaginal herpes simplex virus type 2 infection. Antimicrob Agents Chemother. 2005;49:1181–3. doi: 10.1128/AAC.49.3.1181-1183.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bourne N, Bernstein DI, Ireland J, et al. The topical microbicide PRO 2000 protects against genital herpes infection in a mouse model. J Infect Dis. 1999;180:203–5. doi: 10.1086/314853. [DOI] [PubMed] [Google Scholar]
- 38.Fernández-Romero JA, Abraham CJ, Rodriguez A, et al. Zinc acetate/carrageenan gels exhibit potent activity in vivo against high-dose herpes simplex virus 2 vaginal and rectal challenge. Antimicrob Agents Chemother. 2012;56:358–68. doi: 10.1128/AAC.05461-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zacharopoulos VR, Phillips DM. Vaginal formulations of carrageenan protect mice from herpes simplex virus infection. Clin Diagn Lab Immunol. 1997;4:465–8. doi: 10.1128/cdli.4.4.465-468.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bernstein DI, Stanberry LR, Sacks S, et al. Evaluations of unformulated and formulated dendrimer-based microbicide candidates in mouse and guinea pig models of genital herpes. Antimicrob Agents Chemother. 2003;47:3784–8. doi: 10.1128/AAC.47.12.3784-3788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roy S, Gourde P, Piret J, et al. Thermoreversible gel formulations containing sodium lauryl sulfate or n-lauroylsarcosine as potential topical microbicides against sexually transmitted diseases. Antimicrob Agents Chemother. 2001;45:1671–81. doi: 10.1128/AAC.45.6.1671-1681.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vibholm L, Reinert LS, Søgaard OS, et al. Antiviral and immunological effects of tenofovir microbicide in vaginal herpes simplex virus 2 infection. AIDS Res Hum Retroviruses. 2012;28:1404–11. doi: 10.1089/AID.2012.0078. [DOI] [PubMed] [Google Scholar]
- 43.Valencia F, Veselenak RL, Bourne N. In vivo evaluation of antiviral efficacy against genital herpes using mouse and guinea pig models. Methods Mol Biol Clifton NJ. 2013;1030:315–26. doi: 10.1007/978-1-62703-484-5_24. [DOI] [PubMed] [Google Scholar]
- 44.Celum C, Baeten JM. Tenofovir-based pre-exposure prophylaxis for HIV prevention: evolving evidence. Curr Opin Infect Dis. 2012;25:51–7. doi: 10.1097/QCO.0b013e32834ef5ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nixon B, Jandl T, Teller RS, et al. Vaginally delivered tenofovir disoproxil fumarate provides greater protection than tenofovir against genital herpes in a murine model of efficacy and safety. Antimicrob Agents Chemother. 2014;58:1153–60. doi: 10.1128/AAC.01818-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith JM, Rastogi R, Teller RS, et al. Intravaginal ring eluting tenofovir disoproxil fumarate completely protects macaques from multiple vaginal simian-HIV challenges. Proc Natl Acad Sci USA. 2013;110:16145–50. doi: 10.1073/pnas.1311355110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mesquita PMM, Rastogi R, Segarra TJ, et al. Intravaginal ring delivery of tenofovir disoproxil fumarate for prevention of HIV and herpes simplex virus infection. J Antimicrob Chemother. 2012;67:1730–8. doi: 10.1093/jac/dks097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turpin JA. Topical microbicides to prevent the transmission of HIV: formulation gaps and challenges. Drug Deliv Transl Res. 2011;1:194–200. doi: 10.1007/s13346-011-0034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lard-Whiteford SL, Matecka D, O'Rear JJ, et al. Recommendations for the nonclinical development of topical microbicides for prevention of HIV transmission: an update. J Acquir Immune Defic Syndr. 2004;36:541–52. doi: 10.1097/00126334-200405010-00001. [DOI] [PubMed] [Google Scholar]
- 50.Mauck C, Rosenberg Z, Van Damme L, et al. Recommendations for the clinical development of topical microbicides: an update. AIDS Lond Engl. 2001;15:857–68. doi: 10.1097/00002030-200105040-00006. [DOI] [PubMed] [Google Scholar]