Figure 1. CRABPII is a deacetylation substrate of SIRT1.
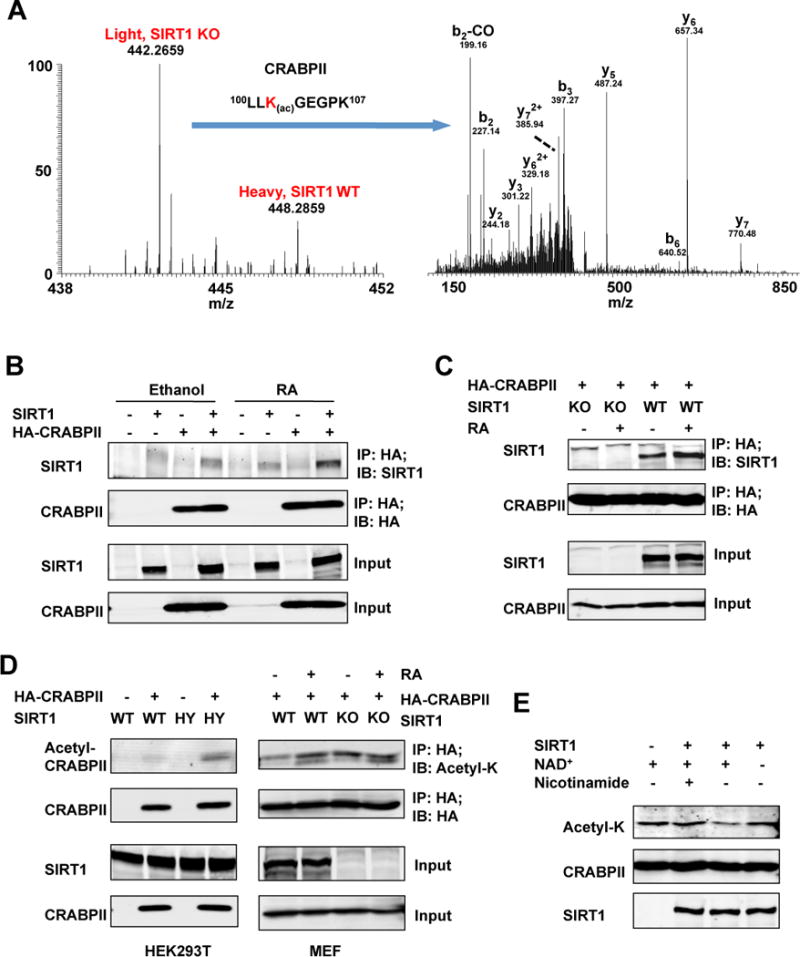
(A) Identification and SILAC quantification of K102 Lys acetylation site on CRABPII. Left panel represents the relative abundances of K102 Lys acetylation sites in SIRT1 KO (light label) and WT (heavy label) MEFs. Right panel shows the MS/MS spectrum that identified “LLK(ac)GEGPK” on CRABPII. b and y ions represent peptide backbone fragmentations containing N- or C- terminus, respectively. Please also see Table S1.
(B) The RA treatment enhances the interaction between HA-CRABPII and SIRT1 in HEK293T cells.
(C) The RA treatment enhances the interaction between CRABPII and endogenous SIRT1 in MEFs.
(D) SIRT1 deficiency increases acetylation levels of CRABPII in HEK293T cells (left panel), and the RA treatment induces the acetylation of CRABPII in WT but not SIRT1 KO MEFs (right panel).
(E) SIRT1 deacetylates CRABPII in vitro. Affinity purified acetylated HA-CRABPII proteins were incubated with or without recombinant SIRT1 protein in buffers containing indicated chemicals as described in Experimental Procedures.
