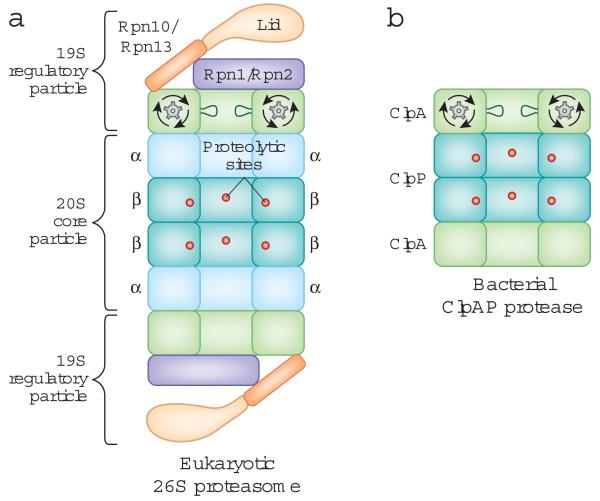
Figure 1.
The overall structure of the eukaryotic proteasome and the bacterial ClpAP protease.
The two proteases have a common architecture. The protease sites are buried in an internal chamber of the core particle, which is capped by regulatory particles that control access to the proteolytic chamber and contain an ATPase hexameric ring.
A: Side-on cross-section of the eukaryotic 26S proteasome. The 20S core particle is flanked by 19S regulatory particles, and the proteolytic sites are located in the β-rings of the 20S core particle. The scaffold proteins Rpn1 and Rpn2, the ubiquitin receptors Rpn10 and Rpn13, and the loops lining the ATPase ring are depicted. Only one set of loops is shown for clarity.
B: Side-on cross-section of the ClpAP protease from Escherichia coli. ClpP contains the proteolytic sites and is capped on both ends by the ClpA ATPase ring.