Figure 1. Reversible lysine acetylation in the PH domain regulates Akt activation.
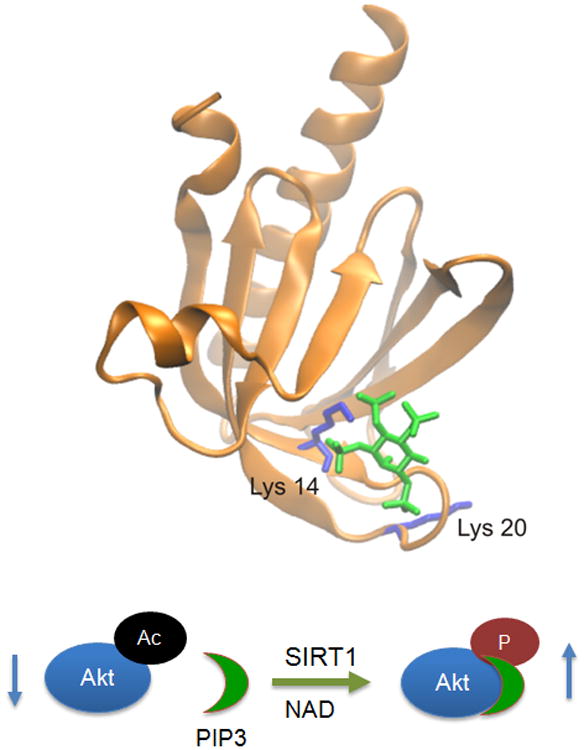
Upper panel: crystal structure of the PH domain of Akt. Acetylated lysine residues (Lys14 and Lys20) are shown in purple and PIP3 in green. Both lysines are in close proximity to the binding pocket for PIP3. Lower panel shows schematic activation of Akt by SIRT1. Lysine acetylation of the PH domain makes Akt incapable to bind to PIP3, leading to inactivation of the kinase. SIRT1-dependent deacetylation promotes Akt-PIP3 binding and hence phosphorylation and activation of Akt by the upstream kinases (for details see ref 9).
