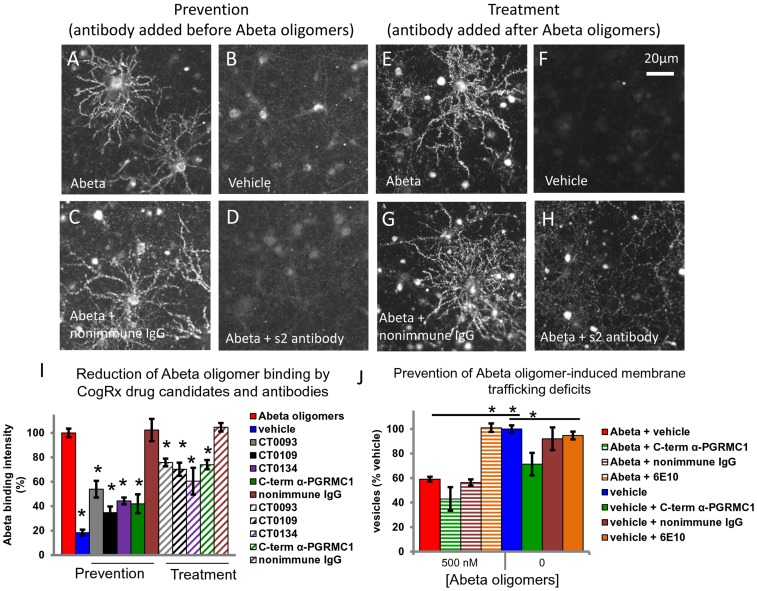
Figure 6. C-terminal antibodies directed against the C-terminus of PGRMC1 prevent (A–D) and displace (E–H) Abeta oligomer binding to neurons and glia.
Abeta oligomers bind to a subset of neurons and glia in mature hippocampal primary neurons 21DIV (A, E, red bar in I) compared to vehicle-treated (no Abeta) cultures (B, F, blue bar in I). Graphs in I are average of 3 experiments (avg. intensity of Abeta oligomer puncta + S.E.M., expressed as a percentage of Abeta oligomer-treated condition, difference in binding intensity vs. Abeta oligomer condition *p<0.05, Student's t-test). Abeta oligomer binding to cultured neurons is significantly reduced in the presence of C-terminal antibody to sigma-2/PGRMC1 regardless of whether it is added before (D, green bar in I [prevention], 58% reduction) or after (H, green hatched bar in I [treatment], 26% reduction) oligomers. This suggests that oligomers are competitively displaced from receptors at synaptic sites. Non-immune IgG (C, G and maroon bars in I) and an N-terminal antibody to sigma-2/PGRMC1 (data not shown) cannot reduce oligomer binding under either condition. J Effects of antibodies on membrane trafficking rate in the presence or absence of Abeta oligomers (expressed as a percentage of vehicle-treated in the absence of Abeta, difference in trafficking rate vs. Abeta oligomer- or vehicle-treated condition *p<0.05, Student's t-test). The C-terminal antibody directed against amino acids 185–195 in sigma-2/PGRMC1 does not rescue oligomer-induced deficits, but induces trafficking deficits on its own in the absence of Abeta oligomers, pointing to a critical role of this protein in normal membrane trafficking.