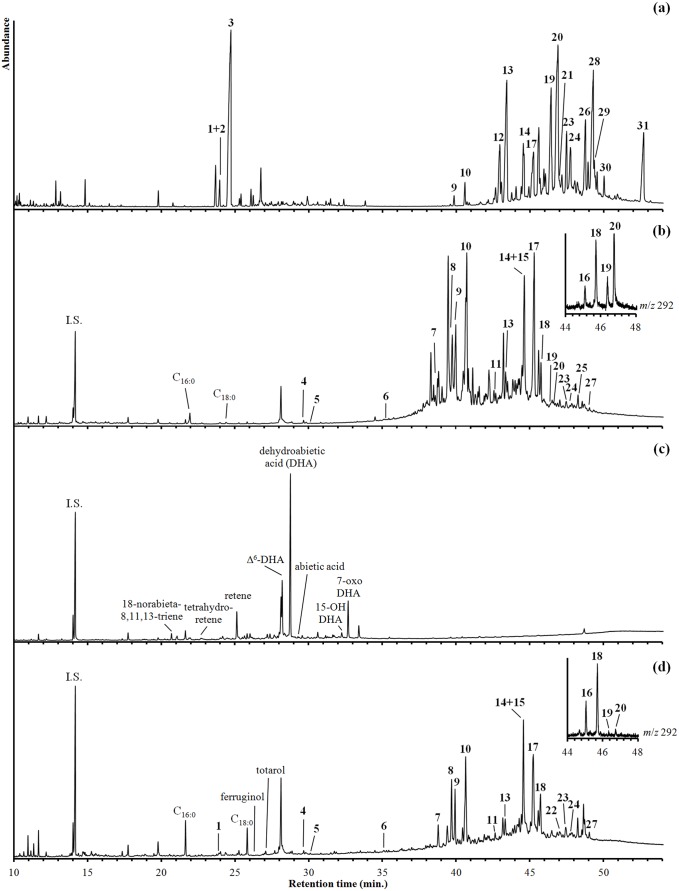
Figure 2. GC-MS chromatograms of silylated extracts of (a) modern Boswellia carterii resin, (b) sample R9, (c) sample Q10 and (d) sample Q11.
Insets in (b) and (d) show partial m/z 292 chromatograms. Legend to compound labels: I.S. = internal standard, C16∶0 = palmitic acid, C18∶0 = stearic acid, DHA = dehydroabietic acid, 1 = serratol (free OH), 2 = incensol (free OH), 3 = incensol (OTMS), 4 = des-A-ursa-5(10),12-diene, 5 = des-A-26,27-dinorursa-5,7,9,11,13-pentaene, 6 = 1,9-dimethylchrysene, 7 = 24-noroleana-3,9(11),12-triene, 8 = 24-norursa-3,9(11),12-triene, 9 = 24-noroleana-3,12-diene, 10 = 24-norursa-3,12-diene, 11 = 24,25,26,27-tetranorursa-1,3,5(10),6,8,11,13-heptaene, 12 = epi-β-amyrin, 13 = epi-α-amyrin, 14 = β-amyrenone, 15 = 24-norursa-3,12-dien-11-one, 16 = Δ2-α-boswellic acid, 17 = α-amyrenone, 18 = Δ2-β-boswellic acid, 19 = α-boswellic acid, 20 = β-boswellic acid, 21 = lupeolic acid, 22 = 2,9-dimethylpicene, 23 = β-elemonic acid, 24 = β-elemolic acid, 25 = 11-keto-α-amyrin, 26 = 3-O-acetyl-α-boswellic acid, 27 = 11-keto- Δ2-β-boswellic acid, 28 = 3-O-acetyl-β-boswellic acid, 29 = 3-O-acetyl-lupeolic acid, 30 = 11-keto-β-boswellic acid, 31 = 3-O-acetyl-11-keto-β-boswellic acid.