Abstract
Cardiac dysfunction is frequently observed in patients with cirrhosis, and has long been linked to the direct toxic effect of alcohol. Cirrhotic cardiomyopathy (CCM) has recently been identified as an entity regardless of the cirrhosis etiology. Increased cardiac output due to hyperdynamic circulation is a pathophysiological hallmark of the disease. The underlying mechanisms involved in pathogenesis of CCM are complex and involve various neurohumoral and cellular pathways, including the impaired β-receptor and calcium signaling, altered cardiomyocyte membrane physiology, elevated sympathetic nervous tone and increased activity of vasodilatory pathways predominantly through the actions of nitric oxide, carbon monoxide and endocannabinoids. The main clinical features of CCM include attenuated systolic contractility in response to physiologic or pharmacologic strain, diastolic dysfunction, electrical conductance abnormalities and chronotropic incompetence. Particularly the diastolic dysfunction with impaired ventricular relaxation and ventricular filling is a prominent feature of CCM. The underlying mechanism of diastolic dysfunction in cirrhosis is likely due to the increased myocardial wall stiffness caused by myocardial hypertrophy, fibrosis and subendothelial edema, subsequently resulting in high filling pressures of the left ventricle and atrium. Currently, no specific treatment exists for CCM. The liver transplantation is the only established effective therapy for patients with end-stage liver disease and associated cardiac failure. Liver transplantation has been shown to reverse systolic and diastolic dysfunction and the prolonged QT interval after transplantation. Here, we review the pathophysiological basis and clinical features of cirrhotic cardiomyopathy, and discuss currently available limited therapeutic options.
Keywords: Cirrhosis, Cardiomyopathy, Pathogenesis, Hyperdynamic circulation, Diastolic dysfunction
Core tip: Currently, little is known about the pathogenesis, diagnostic parameters and therapeutic principles of the cirrhotic cardiomyopathy. Increased cardiac output due to hyperdynamic circulation seems to be a pathophysiological hallmark of the disease. The main clinical features of cirrhotic cardiomyopathy include attenuated systolic contractility in response to physiologic or pharmacologic strain, diastolic dysfunction, electrical conductance abnormalities and chronotropic incompetence. Here, we review the pathophysiological basis and clinical features of cirrhotic cardiomyopathy, and discuss currently available therapeutic options.
INTRODUCTION
Liver cirrhosis is associated with a wide range of cardiovascular abnormalities. Cardiac dysfunction in cirrhotic patients was first described in patients with alcoholic cirrhosis. Thus, almost half a century ago, Kowalski and Abelmann[1] described a hyperdynamic circulation with high cardiac output, decreased arterial pressure and total peripheral resistance in patients with alcoholic cirrhosis. For many following years, cirrhosis-associated cardiac impairment was therefore ascribed to the direct toxic effect of alcohol.
The term cirrhotic cardiomyopathy (CCM) was first introduced more than 3 decades ago, and is defined as chronic cardiac dysfunction in cirrhotic patients in the absence of known cardiac disease, irrespective of the etiology of cirrhosis[2]. Specific diagnostic criteria for CCM have recently been formulated by an international expert consensus committee (Figure 1). Besides increased cardiac output and low systolic blood pressure due to peripheral vasodilatation, frequent cardiac changes during CCM include systolic and/or diastolic dysfunction, electrophysiological abnormalities and chronotropic incompetence. Overt heart failure is not a typical feature of CCM.
Figure 1.
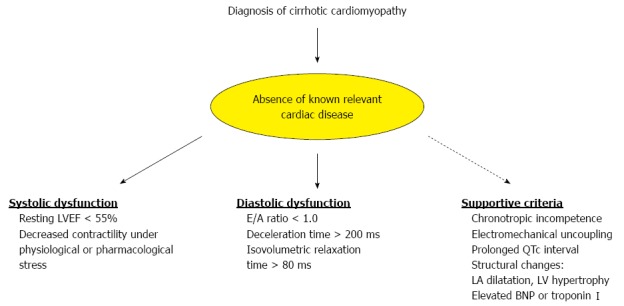
Diagnostic criterion for cirrhotic cardiomyopathy, as defined by the expert consensus committee at the World Congress of Gastroenterology in Montreal, Canada in 2005. LA: Left atria; LV: Left ventricle; EF: Ejection fraction; BNP: Brain natriuretic peptide.
The exact prevalence of CCM remains unknown, because the disease is generally inapparent at rest and becomes manifest under pharmacological or physical stress. Electrocardiographic changes, such as QT prolongation or diastolic dysfunction, are present in the majority of patients with moderately or severely advanced liver failure (Child-Pugh stage B or C)[3]. Generally, cardiomyopathy worsens with the progression of the underlying liver failure.
The following review is a brief update on the pathogenesis of the disease, its clinical implication and management.
PATHOGENESIS OF CCM
The underlying mechanisms involved in CCM are complex and involve interplay of multiple neurohumoral and cellular systems. Current thinking focuses on the increased cardiac output due to hyperdynamic circulation as the key pathogenetic event in CCM. Further studies demonstrated that cardiac contractile function is also adversely affected by cirrhosis, especially when cirrhotic patients are exposed to stress.
CCM predominantly involves systemic multi-factorial cellular, neuronal and humoral signaling pathways. These include the impaired β-receptor and calcium signaling, altered cardiomyocyte membrane physiology, elevated sympathetic nervous tone and increased activity of vasodilatory pathways predominantly through the actions of nitric oxide (NO), carbon monoxide and endocannabinoids[4]. In addition, circulating plasma levels of inflammatory and vasoactive molecules such as endothelins, glucagone, vasoactive intestinal peptide, tumor necrosis factor (TNF)-α, prostacycline and natriuretic peptide are usually accumulated in cirrhosis due to concomitant liver insufficiency and the presence of portosystemic collaterals, and, therefore, might be implied in the CCM pathogenesis.
CELLULAR MECHANISMS
β-Receptor and calcium signaling
The β-adrenergic signaling is crucial in modulating cardiac contractility and chronotropy. The possible role of decreased β-adrenergic receptor density in cirrhosis was first reported by Gerbes et al[5] more than 2 decades ago. Since then β-receptor-mediated pathways have extensively been investigated in CCM. Indeed, the β-adrenergic receptor impairment with a decrease in chronotropic and inotropic responses may be an early sign of CCM[6]. This is likely due to a reduction in both receptor density and function, and is found virtually in all patients with CCM.
In an experimental cirrhosis model, decreased expression of β-receptor density, G-protein subunits Gs and Gi2α with attenuated cAMP generation was reported by several groups[5,7,8]. It was also demonstrated that β-adrenergic receptors were desensitized in vivo[6]. Interestingly, blunted muscarinic responsiveness in cirrhotic myocardium was also attributed to the impaired β-adrenergic pathway[9].
Alterations in the fluidity and biochemical properties of the cellular membrane with increased cholesterol/phospholipids ratio may cause the diminished β-receptor function too, and thus contribute to the pathogenesis of cardiac contractility in cirrhosis[10]. Indeed, abnormal cell membrane fluidity was detected in cardiac tissue[11], erythrocytes[12], kidneys[13] and liver[14] in cirrhosis. On the other hand, the impaired β-receptor signaling in CCM may also be associated with the increased sympathetic tone, a phenomenon frequently observed in end-stage liver disease. For example, Moreau et al[15] showed that the central α-adrenergic agonist clonidine significantly reduced plasma norepinephrine levels and decreased hyperdynamic circulation in cirrhotic patients (Table 1). Consistently, β-receptor antagonists reduce cardiac output in cirrhotic patients by lowering portal pressure and portal flow[16]. In this regard, non-selective β-blockers such as propranolol, nadolol and timolol are more effective than selective β1-blockers in reducing the hepatic venous pressure gradient[17].
Table 1.
Hemodynamic and echocardiographic changes typically observed in cirrhotic cardiomyopathy
| Increased cardiac output and blood volume |
| Decreased left ventricular afterload due to peripheral vasodilation |
| Enhanced sympathetic nervous activity |
| Left ventricular hypertrophy |
| Left atrial enlargement |
| Elevated left ventricular end-diastolic diameter |
| Impairment of diastolic function |
| Evident systolic dysfunction only during stress |
β-adrenergic stimulation or excitation-contraction coupling leads to the activation of various calcium (Ca2+) related systems that are crucial for cardiac contraction. Therefore, alterations in Ca2+ homeostasis may explain the attenuated contractile responsiveness observed in the cirrhotic myocardium. Indeed, voltage-gated L-type Ca2+ channel protein expression is significantly decreased in cardiomyocytes isolated from cirrhotic rats[18]. Moreover, Ca2+ entry as well as Ca2+-- release were diminished in cardiac myocytes in the biliary cirrhotic rat model.
VASOREGULATORY HUMORAL PATHWAYS
Nitric oxide
Among the vasodilators, most attention has been paid to NO as the key humoral factor implicated in pathogenesis of hyperdynamic circulation. NO is synthesized in vascular endothelium constitutively by NO synthase type 1 (neuronal, nNOS) or type 3 (endothelial, eNOS); however, another isoform, the inducible NO synthase (inducible, iNOS) can be expressed upon stimulation with inflammatory mediators. NO stimulates guanylate cyclase to produce cyclic guanosine monophosphate (cGMP), which phosphorylates protein kinase G to inhibit Ca2+ influx into the cytosol and, thus, eventually causing vasodilation. NO exerts a variety of effects on the cardiovascular system. Whereas NO synthesized by nNOS and eNOS exerts cardioprotective effects through improvement of perfusion and inhibition of apoptosis, iNOS-derived NO has a cardiotoxic effect through the suppression of muscle contractility and induction of apoptosis[19].
Plasma NO levels are consistently increased in cirrhotic patients in response to transient bacteremia and increased levels of endotoxins and cytokines[20]. Enhanced NO release has also been detected in splanchnic vasculature of patients with cirrhosis[21]. In cardiac tissue, significantly higher TNF-α, cGMP and iNOS levels were reported in cardiac homogenates obtained from cirrhotic rats, indicating a possible cytokine - iNOS - cGMP mediated pathway in the pathogenesis of CCM[22]. The same study analyzed further the NO-associated effects on cardiac contractility in isolated left ventricular papillary muscles in response to treatment with the non-specific NOS inhibitor nitro-L-arginine methyl ester (L-NAME). The baseline isoproterenol-stimulated papillary muscle contractile force was shown to be lower than in the control groups. However, when the papillary muscles were pre-incubated with the L-NAME, contractile force increased significantly in the cirrhotic rats. Similar results were previously reported by van Obbergh et al[23] who described a significantly increased ventricular contractility in cirrhotic rat hearts after treatment with the non-specific NOS inhibitor, L-NMMA (N omega-monomethyl-L-arginine).
Together, enhanced NOS activity in cirrhotic myocardium as well as the improvement of myocardial contractility after administration of NOS inhibitors suggest a major participation of NO in CCM.
Carbon monoxide
Carbon monoxide, which is mainly produced through the enzymatic actions of heme oxygenase (HO), seems to have as similar biochemical properties as NO. High cGMP levels through activation of guanylyl cyclase were also attributed to the actions of carbon monoxide[24].
Carbon monoxide acts as a physiological vasodilator in hepatic microcirculation[25]. In contrast, up-regulated inducible HO-1 mRNA expression was detected in the right ventricle in animal model of congestive heart failure[26]. Increased carbon monoxide levels are frequently found in cirrhotic patients. Experimental evidence also suggests this substance may be implicated in CCM pathogenesis. This is largely based on the finding of the elevated HO-1 mRNA and protein expression in left ventricle of cirrhotic rats[27]. Furthermore, treatment of cirrhotic heart with HO inhibitor, zinc protoporphyrin IX, restored the elevated cGMP levels[27].
Endocannabinoids
Endogenous cannabinoids, such as anandamide and 2-arachidonoylglycerol, are involved in a variety of pathological processes in chronic liver disease[28]. Endocannabinoids exert a negative inotropic effect in humans and in animal models through their interaction with the inhibitory G-protein-coupled receptors, CB1 and CB2, leading to the inhibition of adenylate cyclase activity and Ca2+ influx into the cytosol of the cardiomyocytes[29,30]. Enhanced expression of anandamide and up-regulation of the cannabinoid signaling pathway has been linked to the pathogenesis of arterial hypotension in cirrhotic rat models[28,31]. Moreover, anandamide was identified as a selective splanchnic vasodilator in cirrhosis[32].
In a rat model of carbon tetrachloride-induced cirrhosis anandamide tissue levels were markedly increased in both heart and liver[33]. Additionally, injection of the CB1 antagonist acutely increased mean blood pressure and improved parameters of cardiac systolic function in cirrhotic rats. In a rat model of bile duct ligated cirrhosis, the blunted contractile response of isolated left ventricular papillary muscle was restored after pre-incubation with a CB1 antagonist[34], suggesting that CB 1-receptor antagonists might be useful to improve contractile function in CCM.
CLINICAL FEATURES
Most patients with stable liver disease have subtle myocardial impairment that is not or less apparent on routine examination. However, with progression of the liver disease or under physiological or pharmacological strain, the cardiac failure becomes manifest.
Cardiac dysfunction resulting from cirrhosis includes impaired systolic or diastolic function, electrophysiological abnormalities with a prolonged ventricular repolarization (QT interval) and chronotropic incompetence. Although some diastolic alterations may precede the systolic disturbances, both forms of dysfunction may develop simultaneously in cirrhotic patients.
Systolic/diastolic dysfunction
Cirrhotic patients exhibit usually normal to increased left ventricular (LV) ejection fraction at rest. Systolic dysfunction is generally manifested as a blunted increase in cardiac output and decreased contractility with exercise or pharmacological stress. For example, Grose et al[35] reported a submaximal increase in cardiac output following exercise in both alcoholic and non-alcoholic cirrhotic patients compared with controls. Similarly, exercise in patients with cirrhosis caused an appropriate increase in LV end-diastolic pressure but without the expected increase in cardiac index or LV ejection fraction, indicating inadequate ventricular reserve[36]. During exercise, there was reduced aerobic capacity and decreased maximal heart rate compared to controls[36].
The cardiac dysfunction is associated with structural and contractile abnormalities. Thus, an enlargement in LV mass, LV end-diastolic and left atrial volumes was detected by magnetic resonance imaging[37]. Consistent with the radiologic findings, an echocardiographic evaluation of cardiac parameters in cirrhotic patients revealed a significant increase in LV end-diastolic diameter and a reduction in peak systolic velocity and systolic strain rate. Interestingly, similar structural changes have been observed in children with biliary atresia awaiting a liver transplantation[38].
In contrast to systolic impairment, diastolic dysfunction is a prominent feature of CCM[39,40]. It describes an impairment of ventricular relaxation with reduction of the early (E) and late (A) phase of ventricular filling, as recorded by Doppler echocardiography. The underlying mechanism of diastolic dysfunction in cirrhosis is likely due to the increased myocardial wall stiffness caused by myocardial hypertrophy, fibrosis and sub-endothelial edema, and subsequently resulting in high filling pressures of the left ventricle and atrium[4].
Several studies demonstrated the presence of echocardiographic parameters of diastolic dysfunction, such as increased A and E wave velocities and deceleration times along with the decreased E/A ratio in cirrhotic patients, especially in those with ascites[39,41]. In patients with ascites, cardiac function can be additionally worsened due to the upward displacement of the diaphragm and increasing intrathoracic pressure[42]. Subsequently, ascites can further diminish the right atrial and ventricular compliance resulting in reduced filling and diastolic dysfunction of the right heart[43]. Paracentesis has been shown to improve ventricular filling by the preload reduction and by the lowering of the increased basal plasma renin activity, aldosterone, norepinephrine, and epinephrine. However, systolic function is generally not affected by the paracentesis[39].
Transjugular intrahepatic portosystemic shunts (TIPS) ameliorate - at least partially - the hyperdynamic state but can, conversely, aggravate heart function by increased cardiac preload that overstrains the left atrium and the right atrium and ventricle[44]. Indeed, a recent multicenter study investigating TIPS vs large volume paracentesis for treatment of ascites, reported that 12% of the TIPS group developed heart failure compared to none in the paracentesis group[45].
In addition, reduced systolic and diastolic function may have prognostic implications as worsening cardiac failure may be a significant factor in the development of renal vasoconstriction and renal dysfunction including hepatorenal syndrome[46].
Electrophysiologic abnormalities
Experimental and clinical evidence suggests that the altered fluidity of myocardial cell membrane and abnormalities in β-receptor signaling predominantly contribute to the electrophysiologic changes seen in cirrhotic patients. Thus, several transmembrane plasma membrane ion channels such as potassium (K+) and Ca2+ have been shown to be dysfunctional both in cirrhotic subjects and cirrhotic animals[47,48]. Interestingly, both ion channels seem to be predominantly involved in conduction abnormalities in cirrhotic patients[49,50].
One of the most common electrophysiologic changes reported in patients with cirrhosis irrespective of its etiology is a QT interval prolongation detected by electrocardiography. QT prolongation has been reported to occur in 37%-84% of cirrhotic individuals with either alcoholic or nonalcoholic liver disease[50]. QT interval prolongation and variability can affect cardiac rhythm and cause serious rhythm disturbances including ventricular arrhythmias and sudden cardiac death. QT prolongation correlates directly with the severity of the liver disease, as defined by the Child-Pugh score[50]. Moreover, a direct relationship between plasma noradrenalin levels and the corrected QT interval was also reported suggesting that enhanced adrenergic stimulation of myocardial cells may play a significant role in abnormal repolarization[50,51].
Chronotropic incompetence is another consistent finding in alcoholic as well as non-alcoholic cirrhosis, and refers to inability of the sinus node to increase heart rate or contractility after appropriate exercise or pharmacological stimulation. Impaired β-receptor signaling and/or autonomic dysfunction are probably the mechanisms underlying the blunted contractile and chronotropic responsiveness in CCM. Chronotropic incompetence has prognostic relevance too, since it is associated with increased risk of perioperative complications, especially in patients undergoing liver transplantation[52,53].
TREATMENT STRATEGIES
Currently, no specific treatment exists for CCM. Given the pivotal role of the cirrhosis itself in the development of circulatory abnormalities, efforts should be made to effectively treat the underlying cirrhotic disease.
In this respect, the liver transplantation is the only established effective treatment for patients with end-stage liver disease and associated cardiac failure. Liver transplantation has been shown to reverse systolic and diastolic dysfunction and the prolonged QT interval after transplantation[54,55]. Additionally, there is a decrease in cardiac output, heart rate, pulmonary artery pressure, and an increase in arterial blood pressure and systemic vascular resistance following liver transplantation[56,57]. The time course of cardiac function recovery as well as factors determining reversibility of the cardiac abnormalities after transplantation are not yet completely understood. Torregrosa et al[54] reported significant improvement in diastolic and systolic function along with the reduction in myocardial mass between 6 and 12 mo after liver transplantation.
When heart failure becomes evident, treatment principles should be as same as for non-cirrhotic heart failure, which include β-blockers, diuretics and preload/afterload reduction. Diuretics are highly effective in the management of CCM-associated fluid retention. While rapid symptomatic improvement and a decrease in volume overload are achieved with loop diuretics, especially for decompensated heart or liver failure, the long-term therapy with these drugs is associated with several adverse effects, such as increased neurohormonal activation, worsening renal function, and electrolyte disturbances[58,59].
β-blockers may reduce the hyperdynamic load and improve the prolonged QT interval, besides their effects on lowering the portal pressure and in the prevention of variceal bleeding. In patients with portal hypertension, β-blockers can be combined with nitrates, which are known to affect the coronary arteries and also have venodilatory effects leading to preload reduction.
Aldosterone antagonists and ACE inhibitors have beneficial effects in inhibition of the renin-angiotensin-aldosterone system overactivity, reduction of LV dilatation and wall thickness as well as improvement of diastolic function. However, both drug groups have not demonstrated long-term efficacy in the treatment of CCM in clinical setting[60,61]. Moreover, ACE inhibitors should be applied with special caution because of their potential to aggravate the systemic vasodilation. Similarly, the use of cardiac glycosides is currently not warranted, since short-acting cardiac glycosides did not improve cardiac contractility in patients with alcoholic cirrhosis and LV dysfunction[62].
CONCLUSION
Cardiac abnormalities are common in patients with liver cirrhosis, regardless of the etiology, and worsens prognosis in these patients. They include increased cardiac output, low systolic blood pressure, systolic and/or diastolic dysfunction, electrophysiological abnormalities and chronotropic incompetence. Overt cardiac failure is not a prominent feature of cirrhosis. However, cardiac dysfunction becomes more apparent with progression of the underlying liver disease.
Pathogenesis of CCM is multifactorial with major involvement of the impaired β-receptor signaling, altered cardiomyocyte membrane physiology, downregulation of intracellular Ca2+ kinetics and increased activity of vasodilatory pathways through the actions of NO, carbon monoxide and endocannabinoids.
Clinical management of CCM remains uncertain because of lack of the clinical evidence and challenging diagnosis of the disease. To date, there are no proven therapies apart from the liver transplantation, which was shown in some studies to reverse the associated cardiac abnormalities.
Footnotes
P- Reviewer: Fett JD, Rinella ME S- Editor: Qi Y L- Editor: A E- Editor: Ma S
References
- 1.Kowalski HJ, Abelmann WH. The cardiac output at rest in Laennec’s cirrhosis. J Clin Invest. 1953;32:1025–1033. doi: 10.1172/JCI102813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee SS. Cardiac abnormalities in liver cirrhosis. West J Med. 1989;151:530–535. [PMC free article] [PubMed] [Google Scholar]
- 3.Baik SK, Fouad TR, Lee SS. Cirrhotic cardiomyopathy. Orphanet J Rare Dis. 2007;2:15. doi: 10.1186/1750-1172-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu H, Gaskari SA, Lee SS. Cardiac and vascular changes in cirrhosis: pathogenic mechanisms. World J Gastroenterol. 2006;12:837–842. doi: 10.3748/wjg.v12.i6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerbes AL, Remien J, Jüngst D, Sauerbruch T, Paumgartner G. Evidence for down-regulation of beta-2-adrenoceptors in cirrhotic patients with severe ascites. Lancet. 1986;1:1409–1411. doi: 10.1016/s0140-6736(86)91556-4. [DOI] [PubMed] [Google Scholar]
- 6.Lee SS, Marty J, Mantz J, Samain E, Braillon A, Lebrec D. Desensitization of myocardial beta-adrenergic receptors in cirrhotic rats. Hepatology. 1990;12:481–485. doi: 10.1002/hep.1840120306. [DOI] [PubMed] [Google Scholar]
- 7.Ma Z, Miyamoto A, Lee SS. Role of altered beta-adrenoceptor signal transduction in the pathogenesis of cirrhotic cardiomyopathy in rats. Gastroenterology. 1996;110:1191–1198. doi: 10.1053/gast.1996.v110.pm8613009. [DOI] [PubMed] [Google Scholar]
- 8.Ma Z, Zhang Y, Huet PM, Lee SS. Differential effects of jaundice and cirrhosis on beta-adrenoceptor signaling in three rat models of cirrhotic cardiomyopathy. J Hepatol. 1999;30:485–491. doi: 10.1016/s0168-8278(99)80109-3. [DOI] [PubMed] [Google Scholar]
- 9.Jaue DN, Ma Z, Lee SS. Cardiac muscarinic receptor function in rats with cirrhotic cardiomyopathy. Hepatology. 1997;25:1361–1365. doi: 10.1002/hep.510250610. [DOI] [PubMed] [Google Scholar]
- 10.Meddings J. Sucrose--how sweet is it? J Pediatr Gastroenterol Nutr. 1997;24:621–622. doi: 10.1097/00005176-199705000-00025. [DOI] [PubMed] [Google Scholar]
- 11.Ma Z, Meddings JB, Lee SS. Membrane physical properties determine cardiac beta-adrenergic receptor function in cirrhotic rats. Am J Physiol. 1994;267:G87–G93. doi: 10.1152/ajpgi.1994.267.1.G87. [DOI] [PubMed] [Google Scholar]
- 12.Kakimoto H, Imai Y, Kawata S, Inada M, Ito T, Matsuzawa Y. Altered lipid composition and differential changes in activities of membrane-bound enzymes of erythrocytes in hepatic cirrhosis. Metabolism. 1995;44:825–832. doi: 10.1016/0026-0495(95)90233-3. [DOI] [PubMed] [Google Scholar]
- 13.Imai Y, Scoble JE, McIntyre N, Owen JS. Increased Na(+)-dependent D-glucose transport and altered lipid composition in renal cortical brush-border membrane vesicles from bile duct-ligated rats. J Lipid Res. 1992;33:473–483. [PubMed] [Google Scholar]
- 14.Reichen J, Buters JT, Sojcic Z, Roos FJ. Abnormal lipid composition of microsomes from cirrhotic rat liver--does it contribute to decreased microsomal function? Experientia. 1992;48:482–486. doi: 10.1007/BF01928168. [DOI] [PubMed] [Google Scholar]
- 15.Moreau R, Lee SS, Hadengue A, Braillon A, Lebrec D. Hemodynamic effects of a clonidine-induced decrease in sympathetic tone in patients with cirrhosis. Hepatology. 1987;7:149–154. doi: 10.1002/hep.1840070129. [DOI] [PubMed] [Google Scholar]
- 16.Lebrec D. Beta-blockers and portal hypertension, hemodynamic effects and prevention of recurrent gastrointestinal bleeding. Hepatogastroenterology. 1990;37:556–560. [PubMed] [Google Scholar]
- 17.Mills PR, Rae AP, Farah DA, Russell RI, Lorimer AR, Carter DC. Comparison of three adrenoreceptor blocking agents in patients with cirrhosis and portal hypertension. Gut. 1984;25:73–78. doi: 10.1136/gut.25.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ward CA, Liu H, Lee SS. Altered cellular calcium regulatory systems in a rat model of cirrhotic cardiomyopathy. Gastroenterology. 2001;121:1209–1218. doi: 10.1053/gast.2001.28653. [DOI] [PubMed] [Google Scholar]
- 19.Kim YM, Bombeck CA, Billiar TR. Nitric oxide as a bifunctional regulator of apoptosis. Circ Res. 1999;84:253–256. doi: 10.1161/01.res.84.3.253. [DOI] [PubMed] [Google Scholar]
- 20.García-Estañ J, Ortiz MC, Lee SS. Nitric oxide and renal and cardiac dysfunction in cirrhosis. Clin Sci (Lond) 2002;102:213–222. [PubMed] [Google Scholar]
- 21.Albornoz L, Motta A, Alvarez D, Estevez A, Bandi JC, McCormack L, Matera J, Bonofiglio C, Ciardullo M, De Santibañes E, et al. Nitric oxide synthase activity in the splanchnic vasculature of patients with cirrhosis: relationship with hemodynamic disturbances. J Hepatol. 2001;35:452–456. doi: 10.1016/s0168-8278(01)00168-4. [DOI] [PubMed] [Google Scholar]
- 22.Liu H, Ma Z, Lee SS. Contribution of nitric oxide to the pathogenesis of cirrhotic cardiomyopathy in bile duct-ligated rats. Gastroenterology. 2000;118:937–944. doi: 10.1016/s0016-5085(00)70180-6. [DOI] [PubMed] [Google Scholar]
- 23.van Obbergh L, Vallieres Y, Blaise G. Cardiac modifications occurring in the ascitic rat with biliary cirrhosis are nitric oxide related. J Hepatol. 1996;24:747–752. doi: 10.1016/s0168-8278(96)80272-8. [DOI] [PubMed] [Google Scholar]
- 24.Ewing JF, Raju VS, Maines MD. Induction of heart heme oxygenase-1 (HSP32) by hyperthermia: possible role in stress-mediated elevation of cyclic 3’: 5’-guanosine monophosphate. J Pharmacol Exp Ther. 1994;271:408–414. [PubMed] [Google Scholar]
- 25.Suematsu M, Ishimura Y. The heme oxygenase-carbon monoxide system: a regulator of hepatobiliary function. Hepatology. 2000;31:3–6. doi: 10.1002/hep.510310102. [DOI] [PubMed] [Google Scholar]
- 26.Raju VS, Imai N, Liang CS. Chamber-specific regulation of heme oxygenase-1 (heat shock protein 32) in right-sided congestive heart failure. J Mol Cell Cardiol. 1999;31:1581–1589. doi: 10.1006/jmcc.1999.0995. [DOI] [PubMed] [Google Scholar]
- 27.Liu H, Song D, Lee SS. Role of heme oxygenase-carbon monoxide pathway in pathogenesis of cirrhotic cardiomyopathy in the rat. Am J Physiol Gastrointest Liver Physiol. 2001;280:G68–G74. doi: 10.1152/ajpgi.2001.280.1.G68. [DOI] [PubMed] [Google Scholar]
- 28.Bátkai S, Járai Z, Wagner JA, Goparaju SK, Varga K, Liu J, Wang L, Mirshahi F, Khanolkar AD, Makriyannis A, et al. Endocannabinoids acting at vascular CB1 receptors mediate the vasodilated state in advanced liver cirrhosis. Nat Med. 2001;7:827–832. doi: 10.1038/89953. [DOI] [PubMed] [Google Scholar]
- 29.Bonz A, Laser M, Küllmer S, Kniesch S, Babin-Ebell J, Popp V, Ertl G, Wagner JA. Cannabinoids acting on CB1 receptors decrease contractile performance in human atrial muscle. J Cardiovasc Pharmacol. 2003;41:657–664. doi: 10.1097/00005344-200304000-00020. [DOI] [PubMed] [Google Scholar]
- 30.Ford WR, Honan SA, White R, Hiley CR. Evidence of a novel site mediating anandamide-induced negative inotropic and coronary vasodilatator responses in rat isolated hearts. Br J Pharmacol. 2002;135:1191–1198. doi: 10.1038/sj.bjp.0704565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ros J, Clària J, To-Figueras J, Planagumà A, Cejudo-Martín P, Fernández-Varo G, Martín-Ruiz R, Arroyo V, Rivera F, Rodés J, et al. Endogenous cannabinoids: a new system involved in the homeostasis of arterial pressure in experimental cirrhosis in the rat. Gastroenterology. 2002;122:85–93. doi: 10.1053/gast.2002.30305. [DOI] [PubMed] [Google Scholar]
- 32.Domenicali M, Ros J, Fernández-Varo G, Cejudo-Martín P, Crespo M, Morales-Ruiz M, Briones AM, Campistol JM, Arroyo V, Vila E, et al. Increased anandamide induced relaxation in mesenteric arteries of cirrhotic rats: role of cannabinoid and vanilloid receptors. Gut. 2005;54:522–527. doi: 10.1136/gut.2004.051599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bátkai S, Mukhopadhyay P, Harvey-White J, Kechrid R, Pacher P, Kunos G. Endocannabinoids acting at CB1 receptors mediate the cardiac contractile dysfunction in vivo in cirrhotic rats. Am J Physiol Heart Circ Physiol. 2007;293:H1689–H1695. doi: 10.1152/ajpheart.00538.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaskari SA, Liu H, Moezi L, Li Y, Baik SK, Lee SS. Role of endocannabinoids in the pathogenesis of cirrhotic cardiomyopathy in bile duct-ligated rats. Br J Pharmacol. 2005;146:315–323. doi: 10.1038/sj.bjp.0706331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grose RD, Nolan J, Dillon JF, Errington M, Hannan WJ, Bouchier IA, Hayes PC. Exercise-induced left ventricular dysfunction in alcoholic and non-alcoholic cirrhosis. J Hepatol. 1995;22:326–332. doi: 10.1016/0168-8278(95)80286-x. [DOI] [PubMed] [Google Scholar]
- 36.Moller S, Henriksen JH. Cardiopulmonary complications in chronic liver disease. World J Gastroenterol. 2006;12:526–538. doi: 10.3748/wjg.v12.i4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Møller S, Søndergaard L, Møgelvang J, Henriksen O, Henriksen JH. Decreased right heart blood volume determined by magnetic resonance imaging: evidence of central underfilling in cirrhosis. Hepatology. 1995;22:472–478. doi: 10.1002/hep.1840220216. [DOI] [PubMed] [Google Scholar]
- 38.Desai MS, Zainuer S, Kennedy C, Kearney D, Goss J, Karpen SJ. Cardiac structural and functional alterations in infants and children with biliary atresia, listed for liver transplantation. Gastroenterology. 2011;141:1264–1272, 1272.e1-4. doi: 10.1053/j.gastro.2011.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pozzi M, Carugo S, Boari G, Pecci V, de Ceglia S, Maggiolini S, Bolla GB, Roffi L, Failla M, Grassi G, et al. Evidence of functional and structural cardiac abnormalities in cirrhotic patients with and without ascites. Hepatology. 1997;26:1131–1137. doi: 10.1002/hep.510260507. [DOI] [PubMed] [Google Scholar]
- 40.Cazzaniga M, Salerno F, Pagnozzi G, Dionigi E, Visentin S, Cirello I, Meregaglia D, Nicolini A. Diastolic dysfunction is associated with poor survival in patients with cirrhosis with transjugular intrahepatic portosystemic shunt. Gut. 2007;56:869–875. doi: 10.1136/gut.2006.102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valeriano V, Funaro S, Lionetti R, Riggio O, Pulcinelli G, Fiore P, Masini A, De Castro S, Merli M. Modification of cardiac function in cirrhotic patients with and without ascites. Am J Gastroenterol. 2000;95:3200–3205. doi: 10.1111/j.1572-0241.2000.03252.x. [DOI] [PubMed] [Google Scholar]
- 42.Karasu Z, Mindikoğlu AL, Van Thiel DH. Cardiovascular problems in cirrhotic patients. Turk J Gastroenterol. 2004;15:126–132. [PubMed] [Google Scholar]
- 43.Guazzi M, Polese A, Magrini F, Fiorentini C, Olivari MT. Negative influences of ascites on the cardiac function of cirrhotic patients. Am J Med. 1975;59:165–170. doi: 10.1016/0002-9343(75)90350-2. [DOI] [PubMed] [Google Scholar]
- 44.Braverman AC, Steiner MA, Picus D, White H. High-output congestive heart failure following transjugular intrahepatic portal-systemic shunting. Chest. 1995;107:1467–1469. doi: 10.1378/chest.107.5.1467. [DOI] [PubMed] [Google Scholar]
- 45.Ginès P, Uriz J, Calahorra B, Garcia-Tsao G, Kamath PS, Del Arbol LR, Planas R, Bosch J, Arroyo V, Rodés J. Transjugular intrahepatic portosystemic shunting versus paracentesis plus albumin for refractory ascites in cirrhosis. Gastroenterology. 2002;123:1839–1847. doi: 10.1053/gast.2002.37073. [DOI] [PubMed] [Google Scholar]
- 46.Møller S, Henriksen JH. Cirrhotic cardiomyopathy. J Hepatol. 2010;53:179–190. doi: 10.1016/j.jhep.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 47.Moreau R, Lebrec D. Endogenous factors involved in the control of arterial tone in cirrhosis. J Hepatol. 1995;22:370–376. doi: 10.1016/0168-8278(95)80292-4. [DOI] [PubMed] [Google Scholar]
- 48.Ward CA, Ma Z, Lee SS, Giles WR. Potassium currents in atrial and ventricular myocytes from a rat model of cirrhosis. Am J Physiol. 1997;273:G537–G544. doi: 10.1152/ajpgi.1997.273.2.G537. [DOI] [PubMed] [Google Scholar]
- 49.Jiménez W, Arroyo V. Origins of cardiac dysfunction in cirrhosis. Gut. 2003;52:1392–1394. doi: 10.1136/gut.52.10.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bernardi M, Calandra S, Colantoni A, Trevisani F, Raimondo ML, Sica G, Schepis F, Mandini M, Simoni P, Contin M, et al. Q-T interval prolongation in cirrhosis: prevalence, relationship with severity, and etiology of the disease and possible pathogenetic factors. Hepatology. 1998;27:28–34. doi: 10.1002/hep.510270106. [DOI] [PubMed] [Google Scholar]
- 51.Aytemir K, Aksöyek S, Ozer N, Gürlek A, Oto A. QT dispersion and autonomic nervous system function in patients with type 1 diabetes. Int J Cardiol. 1998;65:45–50. doi: 10.1016/s0167-5273(98)00091-6. [DOI] [PubMed] [Google Scholar]
- 52.Figueiredo A, Romero-Bermejo F, Perdigoto R, Marcelino P. The end-organ impairment in liver cirrhosis: appointments for critical care. Crit Care Res Pract. 2012;2012:539412. doi: 10.1155/2012/539412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Umphrey LG, Hurst RT, Eleid MF, Lee KS, Reuss CS, Hentz JG, Vargas HE, Appleton CP. Preoperative dobutamine stress echocardiographic findings and subsequent short-term adverse cardiac events after orthotopic liver transplantation. Liver Transpl. 2008;14:886–892. doi: 10.1002/lt.21495. [DOI] [PubMed] [Google Scholar]
- 54.Torregrosa M, Aguadé S, Dos L, Segura R, Gónzalez A, Evangelista A, Castell J, Margarit C, Esteban R, Guardia J, et al. Cardiac alterations in cirrhosis: reversibility after liver transplantation. J Hepatol. 2005;42:68–74. doi: 10.1016/j.jhep.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 55.Møller S, Henriksen JH. Cirrhotic cardiomyopathy: a pathophysiological review of circulatory dysfunction in liver disease. Heart. 2002;87:9–15. doi: 10.1136/heart.87.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Navasa M, Feu F, García-Pagán JC, Jiménez W, Llach J, Rimola A, Bosch J, Rodés J. Hemodynamic and humoral changes after liver transplantation in patients with cirrhosis. Hepatology. 1993;17:355–360. [PubMed] [Google Scholar]
- 57.Gadano A, Hadengue A, Widmann JJ, Vachiery F, Moreau R, Yang S, Soupison T, Sogni P, Degott C, Durand F. Hemodynamics after orthotopic liver transplantation: study of associated factors and long-term effects. Hepatology. 1995;22:458–465. [PubMed] [Google Scholar]
- 58.Cooper HA, Dries DL, Davis CE, Shen YL, Domanski MJ. Diuretics and risk of arrhythmic death in patients with left ventricular dysfunction. Circulation. 1999;100:1311–1315. doi: 10.1161/01.cir.100.12.1311. [DOI] [PubMed] [Google Scholar]
- 59.McCurley JM, Hanlon SU, Wei SK, Wedam EF, Michalski M, Haigney MC. Furosemide and the progression of left ventricular dysfunction in experimental heart failure. J Am Coll Cardiol. 2004;44:1301–1307. doi: 10.1016/j.jacc.2004.04.059. [DOI] [PubMed] [Google Scholar]
- 60.Pozzi M, Grassi G, Ratti L, Favini G, Dell’Oro R, Redaelli E, Calchera I, Boari G, Mancia G. Cardiac, neuroadrenergic, and portal hemodynamic effects of prolonged aldosterone blockade in postviral child A cirrhosis. Am J Gastroenterol. 2005;100:1110–1116. doi: 10.1111/j.1572-0241.2005.41060.x. [DOI] [PubMed] [Google Scholar]
- 61.Timoh T, Protano MA, Wagman G, Bloom M, Vittorio TJ. A perspective on cirrhotic cardiomyopathy. Transplant Proc. 2011;43:1649–1653. doi: 10.1016/j.transproceed.2011.01.188. [DOI] [PubMed] [Google Scholar]
- 62.Limas CJ, Guiha NH, Lekagul O, Cohn JN. Impaired left ventricular function in alcoholic cirrhosis with ascites. Ineffectiveness of ouabain. Circulation. 1974;49:754–760. doi: 10.1161/01.cir.49.4.755. [DOI] [PubMed] [Google Scholar]


