Abstract
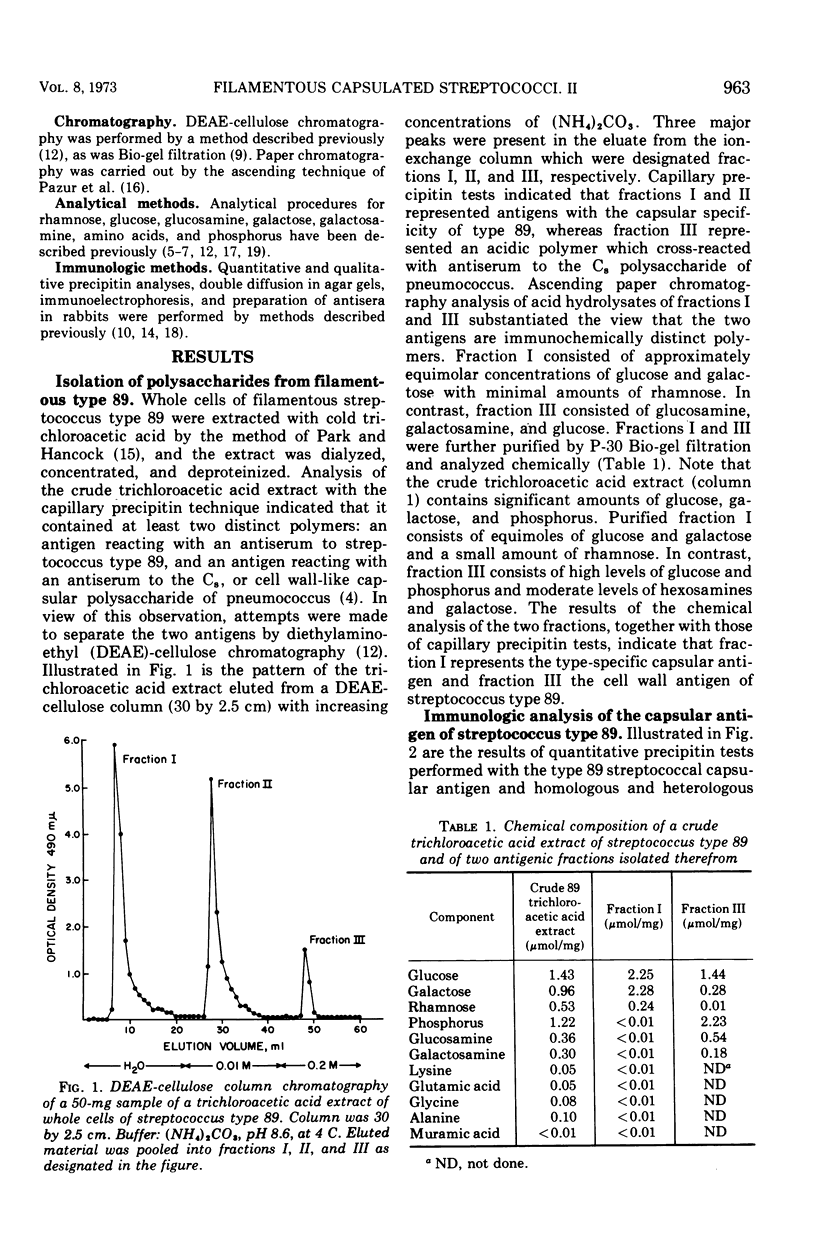
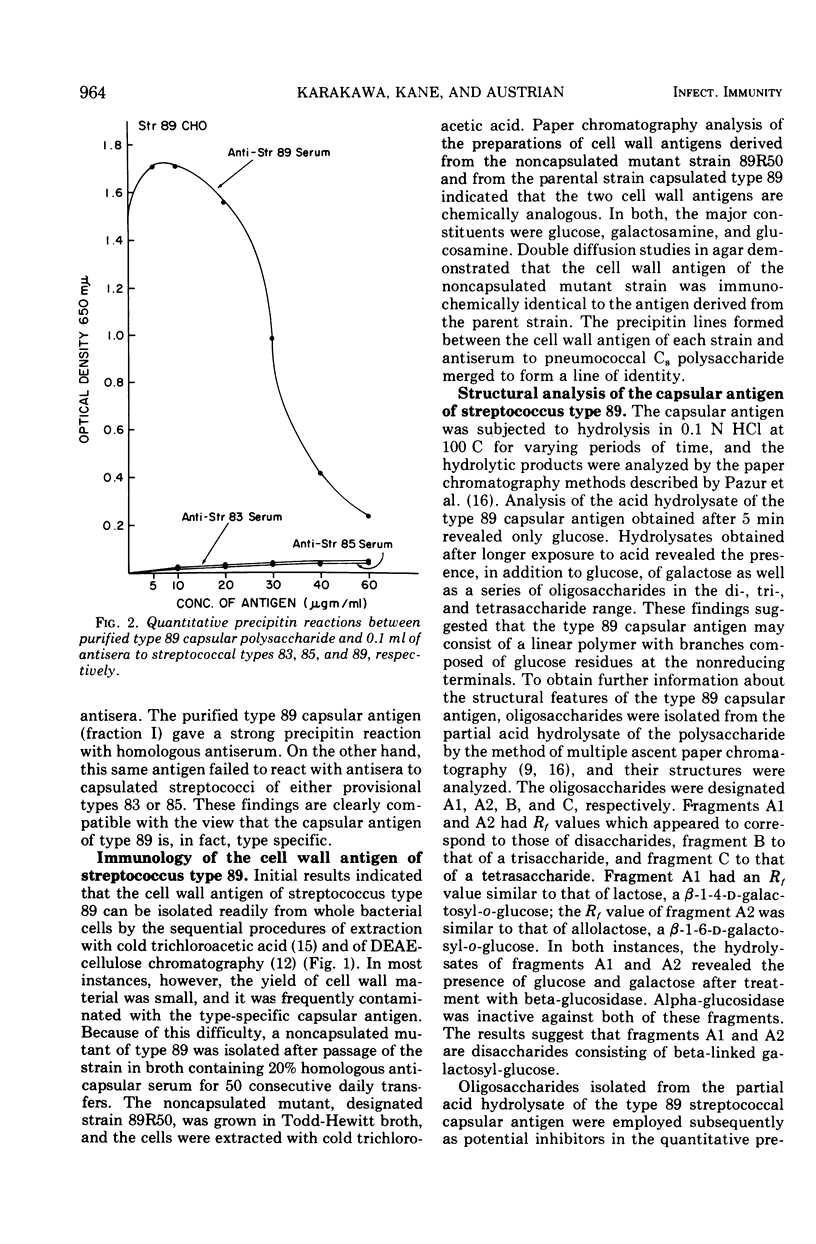
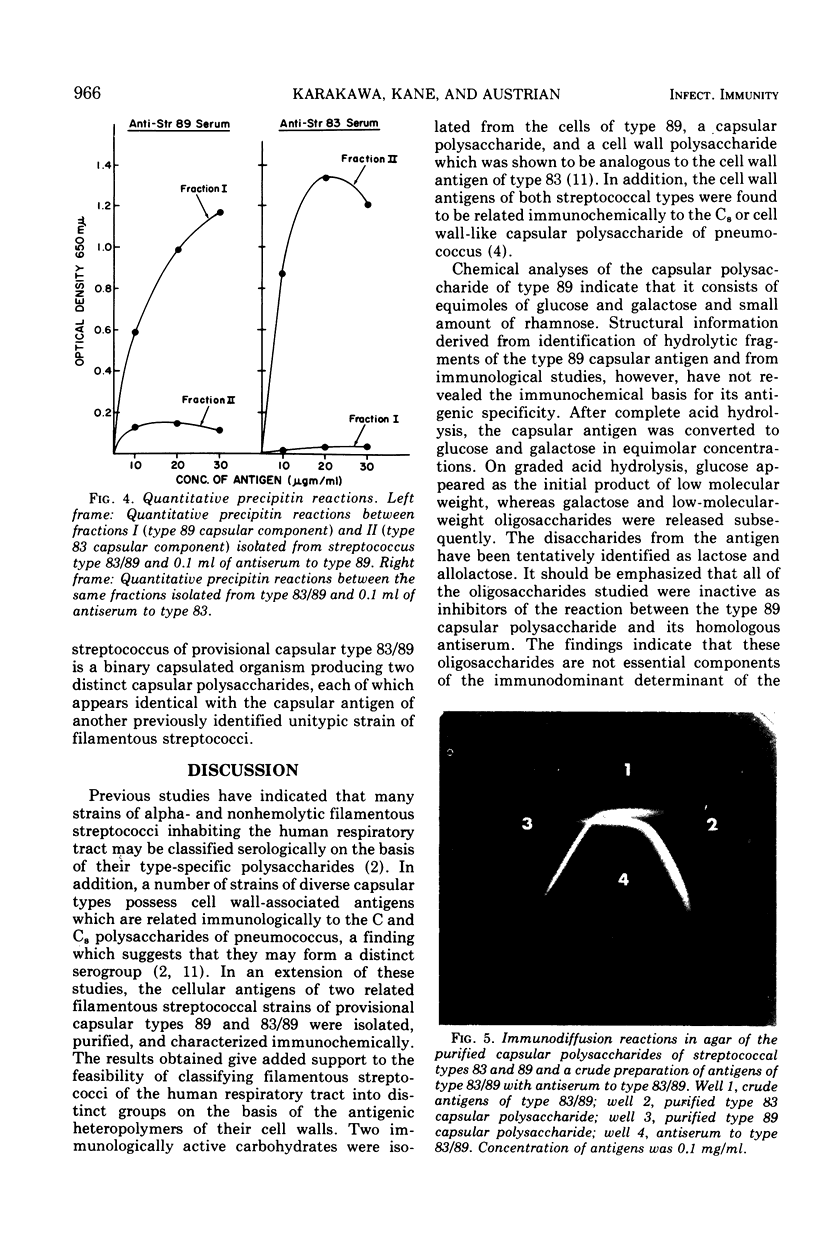
Two immunologically distinct polysaccharides have been isolated from the filamentous alpha-hemolytic streptococcus of provisional capsular type 89, recovered from the human respiratory tract. Chemical analyses indicate that the capsular polysaccharide consists of glucose, galactose, and a small amount of rhamnose, whereas the cell wall-associated polymer contains galactosamine, glucosamine, glucose, and phosphorus. Immunological studies suggest that the capsular polysaccharide is type specific and that the cell wall-associated carbohydrate, which cross-reacts with the C8, or cell wall-like capsular polysaccharide of pneumococcus, may be group specific. A noncapsulated variant of the prototypic streptococcus of provisional type 89 was shown to possess the same cell wall-associated carbohydrate as the strain from which it was derived, but it proved to be poorly antigenic in rabbits. A filamentous capsulated streptococcus reacting with antisera to filamentous streptococci of both provisional capsular types 83 and 89 has been found to produce two capsular polysaccharides, each of which reacts with antibody to one of the aforementioned unitypic strains and represents an unusual binary capsulated streptococcus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUSTRIAN R., BERNHEIMER H. P. Simultaneous production of two capsular polysaccharides by pneumococcus. I. Properties of a pneumococcus manifesting binary capsulation. J Exp Med. 1959 Oct 1;110:571–584. doi: 10.1084/jem.110.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer H. P., Wermundsen I. E., Austrian R. Qualitative differences in the behavior of pneumoncoccal deoxyribonucleic acids transforming to the same capsular type. J Bacteriol. 1967 Jan;93(1):320–333. doi: 10.1128/jb.93.1.320-333.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein D. L., Schiffman G., Bernheimer H. P., Austrian R. Capsulation of pneumococcus with soluble C-like (Cs) polysaccharide. I. Biological and genetic properties of Cs pneumococcal strains. J Exp Med. 1968 Dec 1;128(6):1385–1400. doi: 10.1084/jem.128.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS S. N., KRAUSE R. M. ANTIGENIC RELATIONSHIPS BETWEEN GROUPS B AND G STREPTOCOCCI. J Exp Med. 1964 Oct 1;120:629–637. doi: 10.1084/jem.120.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORSFALL F. L., Jr Studies on non-hemolytic streptococci isolated from the respiratory tract of man; the antigenic basis for type specific reactions with streptococcus salivarius and non-levan-forming streptococci. J Exp Med. 1951 Mar;93(3):229–245. doi: 10.1084/jem.93.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane J. A., Karakawa W. W., Pazur J. H. Glycans from streptococcal cell walls: structural features of a diheteroglycan isolated from the cell wall of Streptococcus bovis. J Immunol. 1972 May;108(5):1218–1226. [PubMed] [Google Scholar]
- Karakawa W. W., Kane J. A. Characterization of the surface antigens of Staphylococcus aureus, strain K-93M. J Immunol. 1972 May;108(5):1199–1208. [PubMed] [Google Scholar]
- Karakawa W. W., Krause R. M. Studies on the immunochemistry of streptococcal mucopeptide. J Exp Med. 1966 Aug 1;124(2):155–171. doi: 10.1084/jem.124.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakawa W. W., Lackland H., Krause M. An immunochemical analysis of bacterial mucopeptides. J Immunol. 1966 Dec;97(6):797–804. [PubMed] [Google Scholar]
- McCARTY M., LANCEFIELD R. C. Variation in the group-specific carbohydrate of group A streptococci. I. Immunochemical studies on the carbohydrates of variant strains. J Exp Med. 1955 Jul 1;102(1):11–28. doi: 10.1084/jem.102.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK J. T., HANCOCK R. A fractionation procedure for studies of the synthesis of cell-wall mucopeptide and of other polymers in cells of Staphylococcus aureus. J Gen Microbiol. 1960 Feb;22:249–258. doi: 10.1099/00221287-22-1-249. [DOI] [PubMed] [Google Scholar]
- Pazur J. H., Anderson J. S., Karakawa W. W. Glycans from streptococcal cell walls. Immunological and chemical properties of a new diheteroglycan from Streptococcus faecalis. J Biol Chem. 1971 Mar 25;246(6):1793–1798. [PubMed] [Google Scholar]
- RONDLE C. J., MORGAN W. T. The determination of glucosamine and galactosamine. Biochem J. 1955 Dec;61(4):586–589. doi: 10.1042/bj0610586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman G., Bornstein D. L., Austrian R. Capsulation of pneumococcus with soluble cell wall-like polysaccharide. II. Nonidentity of cell wall and soluble cell wall-like polysaccharides derived from the same and from different pneumococcal strains. J Exp Med. 1971 Sep 1;134(3 Pt 1):600–617. doi: 10.1084/jem.134.3.600. [DOI] [PMC free article] [PubMed] [Google Scholar]




