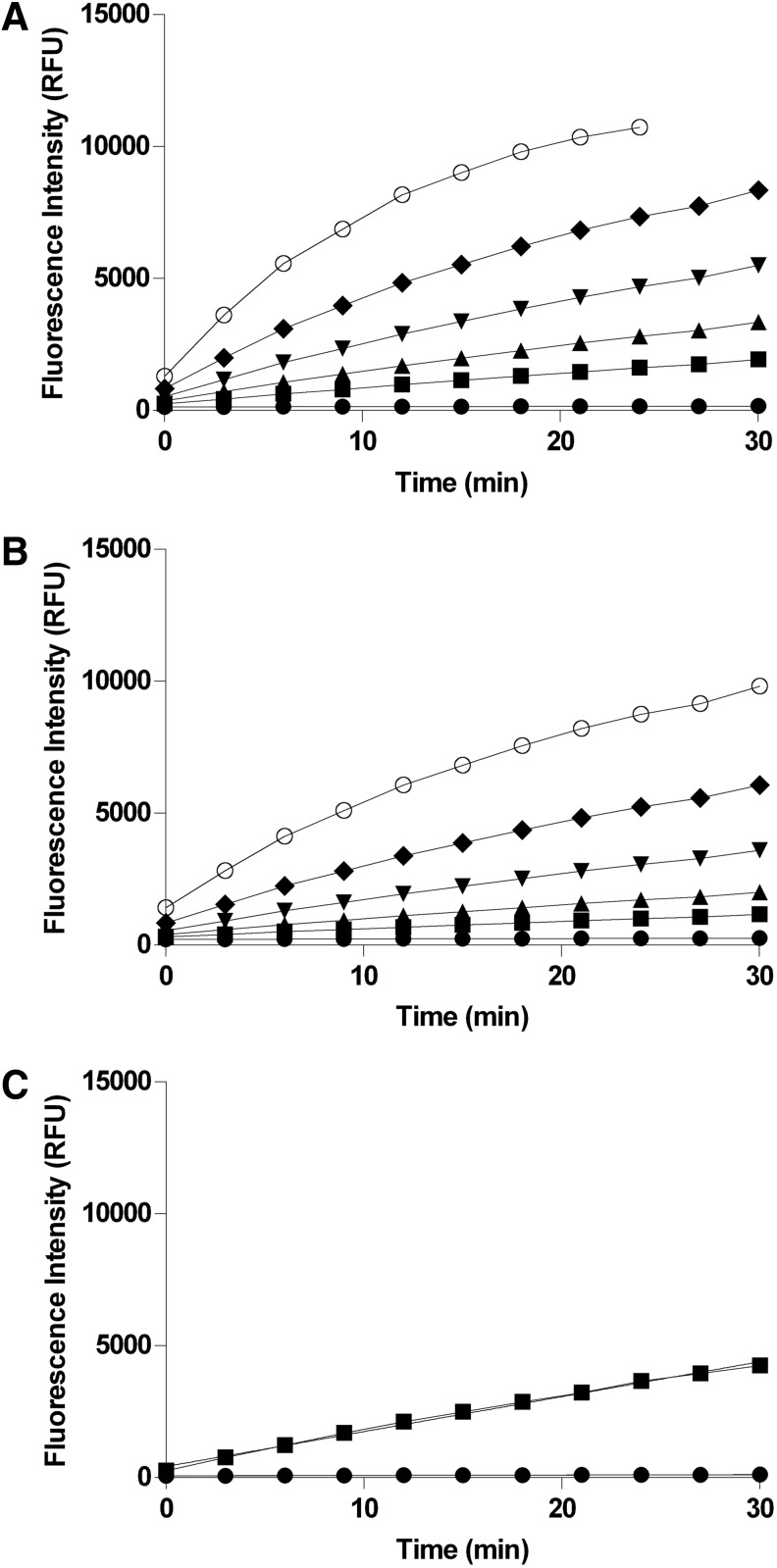
Fig. 2.
Enzyme titration studies. The indicated concentrations of recombinant phosphatase were incubated with either 50 μM (for PP5C) or 100 μM (for PP1α) OMFP substrate in 150 μL reactions at room temperature as described in “Materials and Methods” section. Fluorescence intensity (485-nm excitation/525-nm emission) was measured every 3 min over 30 min with an M5 plate reader. The mean fluorescence intensity±SD of 8 replicates is plotted with GraphPad Prism and linear regression analyses were performed to assess linearity of the progress curves at each concentration. (A) PP5C-dependent progress curves of OMFP hydrolysis at the indicated enzyme concentrations (0 nM [●], 0.2 nM [■], 0.4 nM [▲], 0.8 nM [▼], 1.6 nM [◆], and 3.2 nM [◯]). (B) PP1α-dependent progress curves of OMFP hydrolysis at the indicated enzyme concentrations (0 nM [●], 0.75 nM [■], 1.5 nM [▲], 3 nM [▼], 6 nM [◆], and 12 nM [◯]). (C) PP5C-dependent progress curve at 350 pM PP5C with the addition of 0.1 mM MnCl2 to the reaction buffer. PP5C (350 pM) was incubated at room temperature with 50 μM OMFP in 150 μL reactions. Fluorescence intensity (485-nm excitation/525-nm emission) was measured every 3 min over 30 min with an M5 plate reader. The mean fluorescence intensity±SD of 12 replicates is plotted with GraphPad Prism and linear regression analysis was performed to assess linearity of the progress curve.