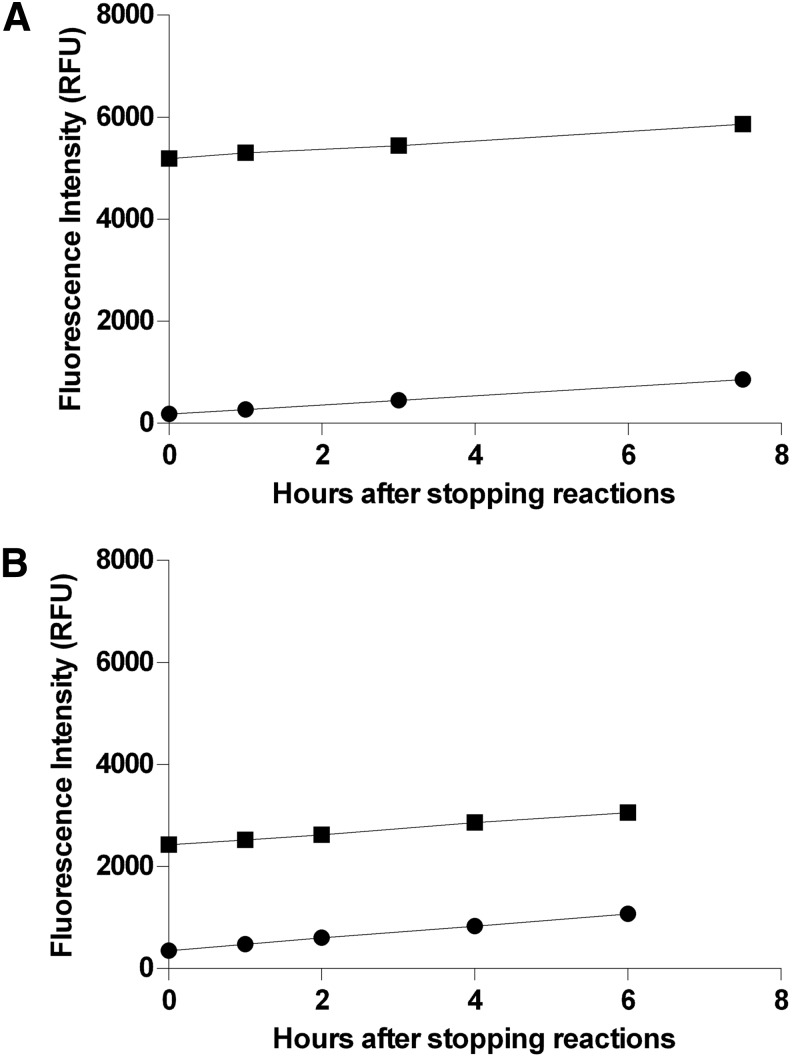
Fig. 7.
Stability of end point fluorescence intensity for controls. Maximum (1% DMSO) and minimum (100 μM cantharidin and 1% DMSO) controls (one 96-well plate per condition) for each phosphatase were prepared as described in “Material and Methods” section. After incubating for 30 min at room temperature, reactions were stopped by the addition of 50 μL of 1 M potassium phosphate (pH 10). Fluorescence intensity (485-nm excitation/525-nm emission) of each replicate was acquired at the indicated times with the M5 plate reader. The first set of measurements (t=0 h) were acquired essentially immediately after stopping the reactions. Plates were subsequently stored in the dark at room temperature except for the brief periods required to acquire FLINT measurements at the indicated times. Due to limitations upon the number of subcolumns in Prism, only data for half of each plate (i.e., 48 replicates for each condition) are displayed here. For both enzymes, after stopping reactions, fluorescence intensities of both maximum- and minimum-signal controls increase steadily at a low rate due to spontaneous hydrolysis of OMFP. (A) Stability of PP5C maximum (■) and minimum (●) controls. (B) Stability of PP1α maximum (■) and minimum (●) controls.