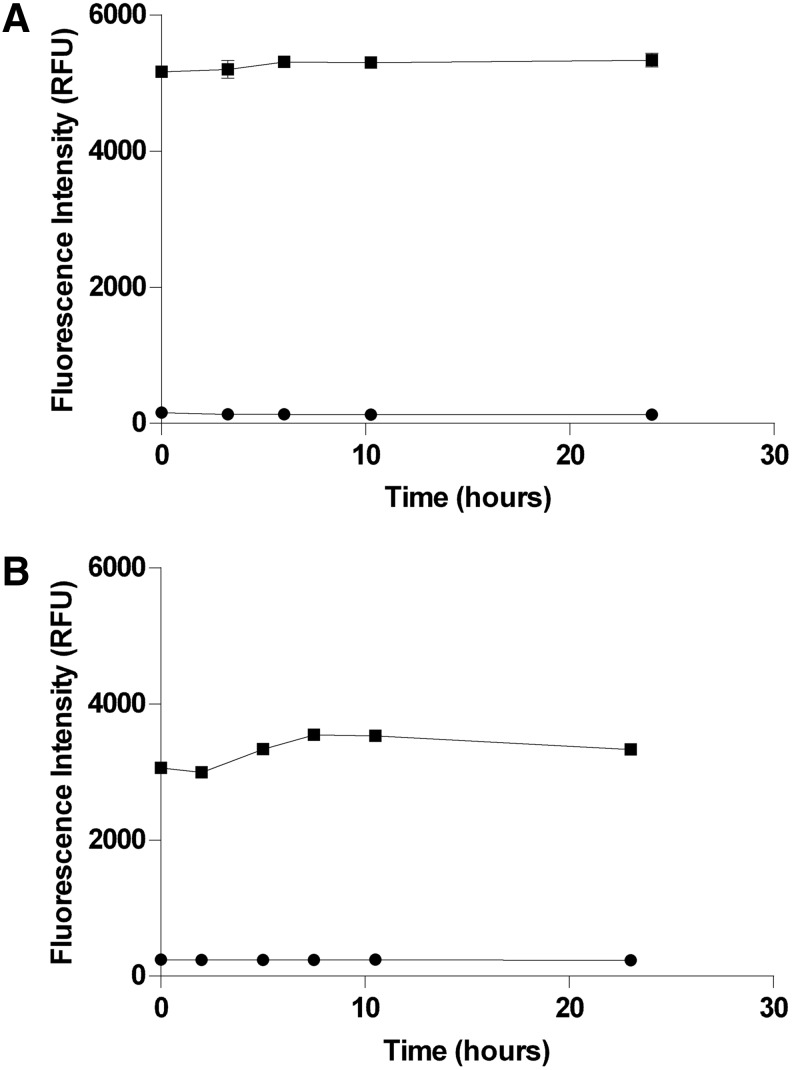
Fig. 8.
Enzyme stability. Solutions of PP5C and PP1α at 1.5× concentration were prepared in the final optimized 1.5× assay buffers as described in “Materials and Methods” section and stored in the dark at room temperature except as noted below. At each of the indicated time points, aliquots of the enzyme solutions were transferred to black 96-well plates. Maximum- (■) and minimum- (●) signal controls were prepared as described (16 replicates of each) at each time point by addition of DMSO and cantharidin, respectively. Reactions were started by addition of OMFP substrate and subsequently stopped by addition of 1 M potassium phosphate after 30-min incubation at room temperature. Fluorescence intensity (485-nm excitation/525-nm emission) measurements of the stopped reactions were then immediately acquired on a SpectraMax M5. (A) PP5C stability. Over the 24-h time frame of this experiment, no deterioration was observed for either the PP5C enzyme activity with OMFP or the sensitivity of PP5C to the control inhibitor cantharidin. (B) PP1α stability. Over the 24-h time frame of this experiment, no significant deterioration was observed for either the PP1α enzyme activity with OMFP or the sensitivity of PP1α to the control inhibitor cantharidin. PP1α activity was observed to be slightly lower during the initial few hours of the experiment. This is thought to be due to poor climate control in the lab that led to relatively lower room temperature during the first part of the experiment.