Abstract
Traditional functional magnetic resonance imaging (fMRI) studies exploit endogenous brain activity for mapping brain activation during “periodic” cognitive/emotional challenges or brain functional connectivity during the “resting state”. Previous studies demonstrated that these approaches provide a limited view of brain function which can be complemented by each other. We hypothesized that graph theory functional connectivity density (FCD) mapping would demonstrate regional FCD decreases between resting-state scan and a continuous “task-state” scan. Forty-five healthy volunteers underwent functional connectivity MRI during resting-state as well as a continuous visual attention task, and standard fMRI with a blocked version of the visual attention task. High-resolution data-driven FCD mapping was used to measure task-related connectivity changes without a priori hypotheses. Results demonstrate that task performance was associated with FCD decreases in brain regions weakly activated/deactivated by the task. Furthermore, a pronounced negative correlation between blood oxygen level-dependent-fMRI activation and task-related FCD decreases emerged across brain regions that also suggest the disconnection of task-irrelevant networks during task performance. The correlation between improved accuracy and stronger FCD decreases further suggests the disconnection of task-irrelevant networks during task performance. Functional connectivity can potentiate traditional fMRI studies and offer a more complete picture of brain function.
Keywords: attention, FCD, fMRI, hub, performance
Introduction
Increasing evidence suggests that cooperative dynamics between and within modular neural networks support the functional organization of the human brain (Fox et al. 2005; Fornito et al. 2012). Traditionally, the functional architecture of the human brain was assessed with correlation analyses of time-varying magnetic resonance imaging (MRI) signals in the resting brain (Fox and Raichle 2007), whereas effective connectivity is used to measure the influence of an activated area over another activated areas during functional magnetic resonance imaging (fMRI) task performance (Friston 2011). The “resting-state” functional connectivity (RSFC) approach is appealing because it requires minimal cooperation/motivation from patients (Fox and Greicius 2010) and is ideal when studying patients with functional deficits that do not fully engage on standard task-based fMRI activation studies. However, the lack of behavioral measures during RSFC data acquisition limits our ability to associate functional connectivity to behavior, and the interpretation of the observed functional connectivity changes/differences between conditions/groups.
The synchronous signals fluctuations in the brain reflect the functional connectivity among brain regions at rest (Biswal et al. 2010) and map into networks that resemble those from task-based functional MRI (fMRI) studies (Smith et al. 2009). For instance, in resting conditions, brain function seems to be intrinsically organized into the dorsal attention and default-mode (DMN) networks, 2 anticorrelated modular systems that show increased and decreased activities during attention-demanding cognitive tasks (Fox et al. 2005). This indicates that the blood oxygen level-dependent (BOLD)-fMRI signals during task-based activation studies represent a combination of spontaneous and task-related signals in the brain (Fox et al. 2006). Thus, the similarity between correlated/anticorrelated and activated/deactivated patterns could reflect an overall predominance of the underlying intrinsic brain activity over the small perturbations caused by cognitive tasks (Raichle 2010).
Since the functional connectivity networks are highly dynamic (Smith et al. 2012), the synchrony of the signal fluctuations is likely to differ for “resting” and “task” conditions. Therefore, task-related modulations of functional connectivity could underlie brain activation during a given task. This hypothesis is supported by previous studies, suggesting that RSFC can predict brain activation during cognitive tasks (Wig et al. 2009; Grigg and Grady 2010; Mennes et al. 2010; Kannurpatti et al. 2012; Tian et al. 2012), and that task performance can affect subsequent RSFC measures (Wang et al. 2012).
However, the small and elusive dynamic functional connectivity changes caused by task performance are largely unknown. When assessed from blocked fMRI data with seed-voxel correlation analyses, the functional connectivity of the task epochs was not different from that of the resting epochs (Fair et al. 2007). Yet, the same analysis for an event-related fMRI task revealed task-related differences in functional connectivity (Fair et al. 2007). More recently, lower fractional amplitude of low-frequency fluctuation (fALFF) in posterior precuneus was reported during an event-related stop signal task than during resting conditions (Zhang and Li 2012). Therefore, the extent to which cognitive performance can alter significantly functional connectivity compared with the resting state is still uncertain. Furthermore, powerful data-driven graph theory measures have not been used to probe functional connectivity measures in task conditions. We hypothesized that, during the performance of a continuous (not blocked) task, the strength of the functional connectivity density (FCD) will be lower than at rest. This hypothesis was based on the observation that task engagement during an event-related fMRI task can attenuate the amplitude of the spontaneous LFFs (Zhang and Li 2012).
To test this hypothesis, we assessed brain activation, with a standard visual attention task (blocked) that requires sustained tracking of moving balls, and brain functional connectivity under 2 different conditions: A standard resting-state condition (eyes open with a fixation cross) and also under a continuous (not blocked) ball-tracking condition. FCD mapping, an ultrafast voxel-wise graph theory method (Tomasi and Volkow 2010), and statistical parametric mapping (SPM) were used to map differences in local (lFCD) and global (gFCD) connectivities between the resting and ball-tracking conditions at a 3-mm isotropic resolution. Subsequent seed-voxel correlation analyses were used to pinpoint the network systems functionally connected with the lFCD and gFCD hubs silenced by the task, and voxel-wise correlations were used to assess the association between ball-tracking accuracy and FCD without a priori hypothesis.
Materials and Methods
Participants
Forty-five healthy right-handed participants (age 30 ± 9 years, education: 13 ± 3 years; mean ± SD; 13 females) signed a written consent approved by the Institutional Review Board at Brookhaven National Laboratory prior to the study. These participants were screened carefully with a detailed medical history, as well as physical and neurological examination. Inclusion criteria were: (1) ability to understand and give informed consent, and (2) 18–55 years of age. Exclusion criteria were: (3) present or past history of neurological, psychiatric, or substance abuse disorder (other than nicotine); (4) the use of psychoactive medications in the past month; (5) the current use of prescription medications; (6) medical conditions that may alter brain function; (7) cardiovascular disease and diabetes; (8) history of head trauma with loss of consciousness of >30 min; (9) history of claustrophobia; (10) excessive weight (body mass index >30 kg/m2); and (11) contraindications to MRI environment (metallic implants/claustrophobia).
Task Paradigm
The object tracking methodology used in this work (Pylyshyn and Storm 1988) is based on a pure attentional processing technique called “visual indexing” (Sears and Pylyshyn 2000). According to visual indexing, a small number of visual objects can be preattentively indexed or tagged and thereby accessed more rapidly by a subsequent attentional process (Pylyshyn 1989; Yantis and Johnston 1990).
During the RSFC scan, the participants rested in the scanner still as possible with their eyes open for 5 min. They were instructed to fixate on a white cross that was displayed at the center of the visual field on a black background. During the “task-state” functional connectivity (TSFC) scan, the participants performed a ball-tracking task that required continuous tracking of 2 moving balls during a single 5-min epoch (Fig. 1). The target balls (2 of 10 balls) were initially highlighted. Then, all balls started to move in a simulated Brownian motion with instantaneous 3°/s angular speed. The subjects' task was to fixate on the center cross and to track the target balls as they moved across the display (12° of the central visual field). Every 11.5 s two balls were highlighted. The subjects' were instructed to press a button if the highlighted balls were the target set. Button press events were used to record performance accuracy and reaction time during the fMRI tasks.
Figure 1.

Study design (A). After a standard “resting-state” functional connectivity (RSFC) scan, the 45 healthy subjects underwent a “task-state” functional connectivity (TSFC) scan were they tracked 2 of the 10 moving balls, continuously during 5 min, followed by a BOLD-fMRI of a blocked version of this visual attention task during 6 min. Time courses of the MRI signal (left) in the SPC and their Fourier spectra (right) during RSFC and TSFC (B) and BOLD fMRI (C) and the canonical HRF used to compute the brain activation (red-yellow: T-score window: 3–10) and deactivation (blue-cyan: T-score window: −3 to −10) patterns for each subject.
During the BOLD-fMRI scan, the subjects performed a blocked version of the continuous ball-tracking task that lasted 6 min and 10 s. This blocked task had 3 cycles interleaving 1-min long “track” epochs with “do not track” epochs, where all 10 balls moved in the same manner but no balls were highlighted. The subjects were instructed to press a button if the highlighted balls were the target set during “track” epochs and to view the balls passively during “do not track” epochs (Fig. 1A). This task activates prefrontal, parietal, and occipital cortices, thalamus, and cerebellum, deactivates the DMN, auditory cortex, and insula (Chang et al. 2004; Tomasi et al. 2004; Tomasi, Goldstein, et al. 2007), and was used to map brain activation in the present study.
The stimuli (movies in “Audio Video Interleave” format) created with Matlab were presented to the subjects on MRI-compatible goggles connected to a personal computer. The display software was synchronized precisely with the MR acquisition using an MRI trigger pulse. All response button events during stimulation were recorded to determine reaction time and performance accuracy (% difference between hits and false alarms). Subjects performed a brief training session (∼3–5 min) of a shortened version of the paradigm outside of the scanner to ensure that they understood and were able to perform the tasks.
MRI Acquisition
Subjects underwent BOLD fMRI in a 4-T whole-body Varian/Siemens MRI scanner using a T2*-weighted single-shot gradient-echo planar imaging sequence with ramp sampling (time echo (TE)/time repetition (TR) = 20/1600 ms, 4 mm slice thickness, 1 mm gap, 33 coronal slices, 64 × 64 matrix size, 3.1 × 3.1 mm in-plane resolution, 90° flip angle) covering the whole brain. Padding was used to minimize motion. Subject motion was determined immediately after each fMRI run to assure minimal head motion <2-mm translations and <2° rotations (Caparelli et al. 2003). Earplugs (28 dB sound pressure-level attenuation; 3M Co., St. Paul, MI, USA), headphones (30 dB sound pressure-level attenuation; Resonance Technology, Inc., Northridge, CA, USA), and a “quiet” acquisition approach were used to minimize the interference effect of scanner noise during fMRI (Tomasi et al. 2005). Anatomical images were collected using a T1-weighted 3D-modified driven equilibrium Fourier transform pulse sequence (Lee et al. 1995; TE/TR = 7/15 ms, 0.94 × 0.94 × 1 mm spatial resolution, axial orientation, 256 readout and 192 × 96 phase-encoding steps, 16 min scan time) and a modified T2-weigthed Hyperecho sequence (Hennig and Scheffler 2001; TE/TR = 42/10 000 ms, echo train length = 16 256 × 256 matrix size, 30 coronal slices, 0.86 × 0.86 mm in-plane resolution, 5 mm thickness, 1 mm gap, 2 min scan time), which were reviewed to rule out gross morphological abnormalities in the brain.
Image Preprocessing
The first 4 volumes in the time series were discarded to avoid nonequilibrium effects in the MRI signal. Subsequent analyses were performed with SPM8 (Wellcome Trust Centre for Neuroimaging, London, UK). A 6-parameter rigid body transformation was used for image realignment, and to correct for head motion. Head motion was <2-mm translations and 2° rotations for all scans. The realigned datasets were normalized to the standard stereotactic space of the Montreal Neurological Institute (MNI) using a 12-parameter affine transformation and a voxel size of 3 × 3 × 3 mm3 (Ashburner and Friston 1999). The interactive data language (IDL, ITT Visual Information Solutions, Boulder, CO, USA) was used for subsequent image preprocessing steps. A multilinear regression approach that used the time-varying realignment parameters (3 translations and 3 rotations) was applied to minimize motion-related fluctuations in the MRI signals (Tomasi and Volkow 2010), and the global signal intensity was normalized across time points. Band-pass temporal filtering (0.01–0.10 Hz) was used to remove magnetic field drifts of the scanner and to minimize physiological noise of high-frequency components (Tomasi and Volkow 2010). Voxels with signal-to-noise (as a function of time) <50 were eliminated to minimize unwanted effects from susceptibility-related signal-loss artifacts. Figure 1B shows representative signal time courses for the TSFC and RSFC scans in the same individual, for a cubic region of interest (ROI, 27 voxels) in the superior parietal cortex (SPC, x y z = 24, −61, 61 mm) that demonstrated strong BOLD-fMRI activation during the blocked ball-tracking task.
Head Motion
Since functional connectivity is particularly sensitive to head motion, we computed the differences in head motion between consecutive image time frames. Specifically, we computed mean motion = (Van Dijk et al. 2012) and framewise displacements (Power et al. 2012) for every time point, i, from head translations () and rotations (αi, βi, γi), the 6-image realignment parameters from SPM2. A radius r = 50 mm, approximately the mean distance from the center of the MNI space to the cortex, was used to convert angle rotations to displacements. Mean motion (RSFC: 0.18 ± 0.08 mm; TSFC: 0.16 ± 0.08 mm) and FD (RSFC: 0.28 ± 0.13 mm; TSFC: 0.26 ± 0.10 mm) were not significantly different for RSFC and TSFC (P > 0.26, t-test) across subjects. A “scrubbing” method was implemented in IDL to remove image time points that could be severely contaminated with motion in the RSFC and TSFC time series (Power et al. 2012). For this purpose, the root mean square variance across voxels (DVARS) of the differences in % BOLD intensity, Ii, between adjacent time points was computed as
where the brackets denote the average across imaging voxels. Image time points with FDi>0.5 mm and DVARSi >0.5% were considered potentially contaminated with motion artifacts and excluded from the time series (Power et al. 2012). The number of time points removed per time series was not different for RSFC (0.2 ± 0.8; mean ± SD) and TSFC (0.3 ± 1.1) scans (P = 0.48, paired t-test). Two sets of preprocessed time series, one with and the other without scrubbing, were evaluated in subsequent analyses to assess the effect of motion correction on task-related differential connectivity.
lFCD and gFCD
The preprocessed RSFC and TSFC time series underwent FCD mapping to compute the strength of the lFCD and gFCD connectivities at 3-mm isotropic resolution. Pearson correlations were used to assess the strength of the functional connectivity, Cij, between voxels i and j in the brain, and a correlation threshold of 0.6 was used to compute the binary undirected connectivity coefficients,
This correlation threshold ensures significant correlation between time-varying signal fluctuations at PFWE < 0.05, minimizes false-positive rate and CPU time, and maximizes sensitivity and dynamic range of the FCD (Tomasi and Volkow 2010). The gFCD, also called “degree centrality” (van den Heuvel et al. 2008; Rubinov and Sporns 2010), was calculated from the N × (N − 1)/2 binary matrices (N = 57 713 voxels) as , using a C-algorithm and parallel computing (Tomasi and Volkow 2011). The lFCD at x0 was computed as the number of elements in the local functional connectivity cluster, , was computed using a “growing” algorithm written in IDL (Tomasi and Volkow 2010). Specifically, a voxel xj was added to the list of voxels functionally connected with xi only if it was adjacent to a voxel that was linked to xi by a continuous path of functionally connected voxels and aij = 1. This calculation was repeated for all voxels that were adjacent to voxels that belonged to the list of voxel functionally connected to xi in an iterative manner until no new voxels could be added to the list. Then, the calculation was initiated for a different xi. A workstation with 2 Intel® Xeon® X5680 processors was used to compute the FCD maps for each subject.
Amplitude of Low-Frequency Fluctuations
The preprocessed RSFC and TSFC time series were also used to map the ALFFs in the whole brain using IDL. The fast Fourier transform was used to compute the ALFF as the average of the power spectrum's square root in the low-frequency bandwidth (0.01–0.06 Hz; Yang et al. 2007) and an fALFF as the ratio between ALFF and the average of the power spectrum's square root in the entire frequency range (0.01–0.31 Hz; Zou et al. 2008).
Seed-Voxel Correlations
Task-related changes in functional connectivity were further assessed with standard seed-voxel correlations in IDL. Seeds with 27 voxels (cubic volume = 0.73 mL) centered at major clusters that demonstrated significant lFCD differences between RSFC and TSFC (Table 1) were used in subsequent seed-voxel correlations assessing the variability of the functional connectivity of these hubs. Pearson correlations were used to compute the strength of the functional connectivity between time-varying signals at the seed locations and those in other brain voxels, and the Fisher transform was used to convert the step distributed correlation coefficients into normally distributed coefficients.
Table 1.
Statistical significance for clusters showing task-related differences in lFCD between the RSFC and TSFC
| Regions | BA/lobe | Voxels | MNI coordinates (mm) |
lFCD [k] |
RSFC > TSFC [T] | BOLD [T] | |||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | RSFC | TSFC | |||||
| Calcarine | 17 | 622 | 3 | −87 | −3 | 16.1 | 11.4 | 6.1 | 3.1 |
| Lingual | 19 | 24 | −57 | −9 | 14.5 | 11.6 | 5.8 | NS | |
| Fusiform | 19 | 27 | −69 | −12 | 14.2 | 11.8 | 5.1 | 3.0 | |
| Precuneus | 7 | 111 | 3 | −66 | 45 | 14.0 | 11.5 | 5.2 | NS |
| Precuneus | 23 | 12 | −63 | 27 | 19.3 | 15.6 | 4.1 | −9.9 | |
| Inferior frontal | 47 | 244 | 45 | 42 | −12 | 10.6 | 7.6 | 5.4 | NS |
| Pars triangularis | 45 | 48 | 39 | 3 | 10.5 | 8.0 | 4.9 | NS | |
| Middle frontal | 8 | 394 | −24 | 21 | 51 | 14.1 | 11.0 | 4.9 | NS |
| Pars triangularis | 45 | −39 | 12 | 27 | 11.0 | 8.7 | 4.9 | NS | |
| Middle frontal | 9 | −30 | 24 | 45 | 15.1 | 11.7 | 4.8 | −3.2 | |
| Cerebellum | Posterior | 767 | 9 | −48 | −42 | 14.7 | 10.7 | 5.0 | NS |
| Cerebellum | Posterior | −6 | −51 | −36 | 16.8 | 12.0 | 4.9 | 3.7 | |
| Cerebellum | Posterior | 0 | −48 | −48 | 11.6 | 9.0 | 4.8 | NS | |
| Superior temporal | 43 | 526 | −63 | −21 | 30 | 13.5 | 9.5 | 4.9 | NS |
| Middle occipital | 19 | −33 | −72 | 30 | 14.0 | 11.1 | 4.4 | −6.5 | |
| Middle occipital | 19 | −39 | −78 | 33 | 16.3 | 12.2 | 4.6 | −9.2 | |
| Angular | 39 | 51 | 51 | −63 | 30 | 14.2 | 10.8 | 4.5 | −4.9 |
| Postcentral | 4 | 103 | 48 | −12 | 27 | 8.6 | 6.4 | 3.5 | NS |
| Precentral | 6 | 54 | 0 | 24 | 13.5 | 10.3 | 4.3 | NS | |
Note: Within-subjects ANOVA. Sample size: 45 healthy subjects.
Bold region labels identify the seeds regions used to compute the correlation analysis reflecting the strength of the functional connectivity with the seed regions.
Statistical Analysis
After spatial smoothing (an 8-mm full-width half-maximum Gaussian kernel), the BOLD-fMRI time series were analyzed with the general linear model in SPM8 to estimate brain activation maps for each subject. Specifically, a box-car design (1 min “track” epochs; 1 min “do not track” baseline epochs; 3 cycles) convolved with the canonical hemodynamic response function (HRF) and a high-pass (cut-off frequency: 1/256 Hz) filter was used to contrast task epochs versus “do not track” baseline epochs. Figure 1C shows the BOLD-fMRI signal time course and the HRF for the SPC ROI for a representative subject. These contrast maps were included in a second-level 1-sample t-test model in SPM8 to map brain activation and deactivation during ball tracking.
Spatial smoothing (8 mm) was also applied to lFCD, gFCD, ALFF, fALFF, RSFC, and TSFC maps to minimize the impact of variable brain anatomy across subjects. Within-subjects analysis of variance (ANOVA) in SPM8 was used to assess lFCD, gFCD, ALFF, and fALFF differences between RSFC and TSFC. A simple regression SPM8 model was used to test the linear association of lFCD, gFCD, ALFF, and fALFF with performance accuracy during the TSFC scan; note that the association with reaction time was not possible because the ball-tracking task does not provide a reliable reaction time measure. Statistical significance was based on a threshold PFWE < 0.05, corrected for multiple comparisons at the cluster level with the random field theory and a family-wise error (FWE) correction. Anatomical labeling was based on the Automated Anatomical Labeling (AAL) atlas and the population-average landmark- and surface-based atlas of the cerebral cortex provided in the installation package of the MRIcron image viewer (http://www.nitrc.org/projects/mricron).
Results
Behavior
During the BOLD-fMRI and TSFC scans, the subjects tracked the balls with similar performance accuracy (BOLD fMRI: 87 ± 2% and TSFC: 87 ± 2%; mean ± SEM) and reaction time (BOLD fMRI: 0.87 ± 0.06 s and TSFC: 0.82 ± 0.05 s). Performance accuracy measures during BOLD fMRI and TSFC were correlated across subjects (R = 0.62; P < 0.0001). Reaction time measures during BOLD fMRI and TSFC did not show significant correlation (R = 0.07).
BOLD-fMRI Patterns
The ball-tracking tasks activated a bilateral network (Fig. 2) that includes parietal, prefrontal, cingulate, and occipital cortices, thalamus, cerebellum and midbrain, and deactivated DMN regions, motor and premotor, superior frontal, posterior occipital, temporal cortices, and posterior insula (Fig. 2), which are consistent with those reported in our previous studies on visual attention (Tomasi et al. 2004, 2006; Tomasi, Chang, et al. 2007; Tomasi, Goldstein, et al. 2007; Tomasi, Volkow, et al. 2009).
Figure 2.
Statistical t-score maps for brain activation (red-yellow) and deactivation (blue-cyan) during the ball-tracking task (top row) as well as for the task-related decreases in local (lFCD; middle row) and global (gFCD; bottom row) functional connectivity densities superimposed on the surface of the Colin human brain template. Independent within-subjects ANOVA for each image modality. Sample size: 45 healthy subjects.
lFCD and gFCD
Consistent with our previous studies (Tomasi and Volkow 2010), the most prominent lFCD hubs were located in posterior cingulate/ventral precuneus parietal, frontal, occipital, and cingulate cortices, insula, thalamus, caudate, and cerebellum, both for RSFC and TSFC. Also consistent with our previous studies (Tomasi and Volkow 2011), the most prominent gFCD hubs were located in posterior cingulum ventral precuneus, angular gyrus, visual cortex, supplementary motor area, globus pallidus, thalamus, and inferior frontal operculum.
The patterns of the task-related differences were similar for lFCD and gFCD (Fig. 2), which demonstrated task-related decreases in visual cortex (BAs 17 and 19), DMN (BA 7), inferior frontal cortex (BAs 45 and 47), superior temporal (BA 43), and the premotor cortex (BA 6). This most likely reflects the overall high correlation between lFCD and gFCD across subjects (R = 0.6 ± 0.2, mean ± SD) in regions that demonstrated task-related FCD decreases (all 57 ROIs in Tables 1–3; RSFC and TSFC included). The lFCD and gFCD were not higher for TSFC than for RSFC in any brain region. The strength of the lFCD was also lower in DMN (BAs 7, 23, and 39), visual (BAs 17 and 19), temporal (BA 43), dorsolateral prefrontal (BA 9), motor and premotor (BAs 4 and 6) and language (BAs 45 and 47) areas, and cerebellum for TSFC than for RSFC (Table 1). The strength of the gFCD in DMN (BAs 7 and 39), visual (BAs 17–19), auditory (BA 22), parietal (BA 40), dorsolateral prefrontal (BAs 9 and 46), premotor (BAs 4 and 6), and language (BAs 44 and 45) areas and cerebellar vermis was lower for TSFC than for RSFC (Table 2). Scrubbing did not alter significantly these results in any brain region [Puncorrected > 0.01, minimal cluster volume = 100 voxels; (RSFC − TSFC) × (scrubbed − unscrubbed) interaction effects; within-subjects ANOVA; Fig. 3].
Table 3.
Statistical significance for the linear association of brain activation, lFCD and gFCD, with accuracy performance
| Regions | BA | Voxels | MNI coordinates (mm) |
Negative slope |
BOLD fMRI [T] | |||
|---|---|---|---|---|---|---|---|---|
| x | y | z | [T] | PFWE | ||||
| BOLD-fMRI versus accuracy | ||||||||
| Precuneus | 7 | 1143 | −3 | −63 | 36 | 4.8 | 0.001 | −9.0 |
| Cuneus | 18 | 9 | −69 | 24 | 4.9 | |||
| Precuneus | 7 | 0 | −69 | 30 | 4.9 | |||
| lFCD versus accuracy | ||||||||
| Lingual | 18 | 420 | 12 | −66 | 0 | 5.3 | 0.003 | −3.4 |
| Lingual | 18 | −3 | −66 | 0 | 4.9 | |||
| Lingual | 18 | −15 | −57 | −6 | 4.7 | |||
| Postcentral | 3 | 359 | 36 | −36 | 54 | 4.8 | 0.006 | 5.5 |
| Postcentral | 3 | 42 | −15 | 33 | 4.0 | |||
| Postcentral | 3 | 36 | −21 | 45 | 3.5 | |||
| Thalamus | 208 | 12 | −15 | 18 | 4.6 | 0.037 | 3.4 | |
| Thalamus | −9 | −12 | 18 | 4.5 | ||||
| Middle occipital | 19 | 188 | −42 | −78 | 9 | 4.1 | 0.049 | 2.7 |
| Middle occipital | 37 | −45 | −69 | 0 | 3.7 | |||
| Inferior occipital | 19 | −57 | −75 | 3 | 3.6 | |||
| gFCD versus accuracy | ||||||||
| Calcarine | 17 | 573 | 6 | −81 | 0 | 6.8 | 0.001 | NS |
| Lingual | 19 | 18 | −63 | −6 | 6.0 | |||
| Lingual | 18 | −15 | −57 | −6 | 4.5 | |||
| Inferior parietal | 40 | 228 | 39 | −54 | 51 | 4.4 | 0.034 | 3.5 |
| Inferior parietal | 40 | 36 | −42 | 54 | 4.3 | |||
| Superior parietal | 7 | 27 | −57 | 54 | 3.9 | |||
Note: Simple regression analyses. Sample size: 45 healthy subjects.
Table 2.
Statistical significance for clusters showing task-related differences in gFCD between the RSFC and TSFC
| Region | BA | Voxels | MNI coordinates (mm) |
gFCD [k] |
RSFC > TSFC [T] | BOLD [T] | |||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | RSFC | TSFC | |||||
| Precuneus | 7 | 378 | 0 | −75 | 33 | 378 | 228 | 5.1 | −9.1 |
| Inferior parietal | 7 | −33 | −75 | 45 | 400 | 156 | 4.8 | −6.7 | |
| Middle temporal | 39 | −45 | −69 | 21 | 370 | 175 | 4.6 | −6.1 | |
| Fusiform | 19 | 1465 | 27 | −60 | −15 | 349 | 145 | 5.6 | NS |
| Calcarine | 17 | 9 | −84 | 3 | 334 | 115 | 5.6 | NS | |
| Lingual | 18 | 12 | −78 | −9 | 370 | 132 | 5.2 | NS | |
| Supramarginal | 40 | 208 | −60 | −42 | 30 | 309 | 130 | 4.9 | NS |
| Postcentral | 22 | −66 | −15 | 18 | 202 | 94 | 4.0 | −3.3 | |
| Supramarginal | 2 | −51 | −24 | 30 | 266 | 125 | 3.6 | NS | |
| Pars opercularis | 44 | 111 | −45 | 9 | 24 | 249 | 124 | 4.2 | 3.1 |
| Precentral | 6 | −54 | 0 | 21 | 303 | 154 | 4.3 | NS | |
| Pars triangularis | 45 | −45 | 27 | 18 | 216 | 121 | 3.8 | NS | |
| Rolandic operculum | 22 | 116 | 63 | −18 | 15 | 264 | 137 | 4.3 | −3.8 |
| Superior temporal | 22 | 57 | −6 | 3 | 331 | 198 | 4.0 | −8.9 | |
| Pars opercularis | 44 | 48 | 9 | 18 | 240 | 133 | 4.0 | 7.4 | |
| Middle frontal | 9 | 84 | 30 | 39 | 42 | 152 | 73 | 3.5 | NS |
| Middle frontal | 46 | 36 | 36 | 30 | 177 | 88 | 4.0 | 5.7 | |
| Middle frontal | 46 | 27 | 33 | 27 | 105 | 54 | 3.1 | 3.9 | |
| Middle frontal | 45 | 54 | −42 | 42 | 18 | 139 | 68 | 4.4 | 4.0 |
| Middle frontal | 45 | −45 | 39 | 30 | 102 | 49 | 2.0 | NS | |
| Vermis | 111 | 3 | −54 | −27 | 510 | 140 | 4.1 | 4.5 | |
| Vermis | 0 | −78 | −30 | 501 | 194 | 3.9 | 4.5 | ||
Note: Within-subjects ANOVA. Sample size: 45 healthy subjects.
Bold region labels identify the seeds regions used to compute the correlation analysis reflecting the strength of the functional connectivity with the seed regions.
Figure 3.
Regions that showed significant (P < 0.001, uncorrected) task-related connectivity decreases (red-yellow) for datasets with and without scrubbing, superimposed on axial views of the human brain.
Most of the regions that showed task-related FCD differences did not show significant activation or deactivation during the blocked BOLD-fMRI task. The overlap between the patterns of task-related lFCD differences and BOLD-fMRI activation (deactivation), computed using a P < 0.001 uncorrected voxel-level threshold, was 5% (3%), relative to the whole-brain volume, and the corresponding nonoverlapping patterns of lFCD differences and BOLD activation (deactivation) occupied 20% and 24% (18%), respectively. Thus, the overlap was minimal and occurred in weakly activated/deactivated brain regions (Fig. 4A). Whereas lFCD hubs in the visual cortex and cerebellum (posterior lobe) showed weak activation, ventral precuneus, the main functional hub in the brain (Tomasi and Volkow 2010), middle occipital and frontal cortices, and angular gyrus showed pronounced deactivation during the blocked BOLD-fMRI task (Table 1). Whereas DMN and auditory regions showed significant BOLD-fMRI deactivation, frontal areas (BA 44–46) and vermis showed significant BOLD-fMRI activation with the task (Table 2). For voxels with strong activation (PFWE < 0.05), the statistical significance of the activation and that of the lFCD differences showed a pronounced negative correlation (R = −0.92; Fig. 4B), in a way that stronger activation was associated with weaker lFCD differences across voxels.
Figure 4.

(A) Nonoverlapping patterns of BOLD-fMRI and task-related lFCD decreases and their overlap. (B) Scatter plot showing the negative correlation of brain activation and task-related lFCD decreases. The lFCD hubs were sorted by their strength into bins of ΔFCD = 1 and the average lFCD and BOLD fMRI values computed within these ΔFCD bins using IDL.
ALFF and fALFF
Follow-up ROI analyses demonstrated that the amplitude of the signal fluctuations (measured as the standard deviation of the signal as a function of time) at the locations of the FCD decreases was 10–25% lower during TSFC than during RSFC (P < 0.002; paired t-test). The signal amplitudes were more pronounced in the lower frequency band (0.01–0.06 Hz) than in the upper frequency band (0.06–0.10 Hz) of the Fourier spectrum. Complementary voxel-wise analyses revealed significant task-related decreases in the amplitude of the signal fluctuations in posterior precuneus (x, y, z = 0, −49, 65 mm), fusiform (x, y, z = 24, −57, −12 mm), and postcentral (x, y, z = −63, −9, 30 mm) gyri (BAs 7, 19, and 43; ALFF), and lingual gyrus (x, y, z = −24, −54, −6 mm; BA 19; fALFF) that were less prominent than the task-related FCD decreases. Task-related increases in ALFF or fALFF overlapped regions that showed task-related decreases in lFCD and gFCD, but were not statistically significant after corrections for multiple comparisons in any brain region.
Seed-Voxel Correlation Analyses
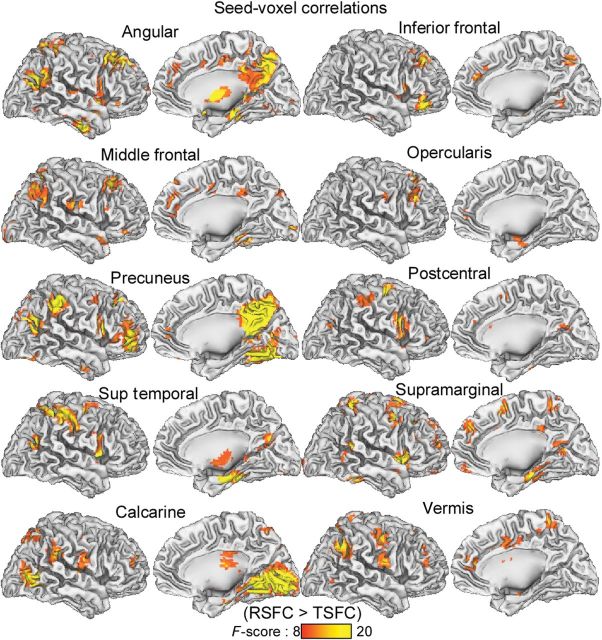
The seed-voxel correlation maps were used to confirm the functional connectivity decreases from RSFC to TSFC in the whole-brain for the 10 major FCD hubs, listed in Tables 1 and 2 (Fig. 5). These voxel-wise analyses at PFWE < 0.05 revealed lower connectivity for TSFC than for RSFC for the “calcarine hub” (x, y, z = 3, −87, −3 mm) with primary and secondary visual cortices and cerebellum (positive connectivity) and with posterior and inferior parietal regions and fusiform gyrus (negative); “precuneus hub” (x, y, z = 3, −66, 45 mm) with bilateral posterior DMN and superior prefrontal regions (positive) and with visual and left language areas (negative); “inferior frontal hub” (x, y, z = 45, 42, −12 mm) with language areas (positive); “middle frontal hub” (x, y, z = −24, 21, 51 mm) with the superior frontal cortex (positive), and with the left occipital cortex (negative); “vermis hub” (x, y, z = 9, −48, −42 mm) with cerebellum and posterior parietal regions (positive); “superior temporal hub” (x, y, z = −63, −21, 30 mm) with the inferior parietal cortex (positive) and with parahippocampal gyrus (negative); “angular hub” (x, y, z = 51, −63, 30 mm) with ventral and lateral parietal, superior prefrontal, and left inferior temporal regions, anterior thalamus, cerebellum, and inter posterior cingulum (positive) and with temporal pole and anterior insula (negative); “postcentral hub” (x, y, z = 48, −12, 27 mm) with right temporal cortex (positive) and with left putamen (negative); “supramarginal hub” (x, y, z = −60, −42, 30 mm) with SPC, insula, right supramarginal gyrus, and caudate (positive); “opercularis hub” (x, y, z = −45, 9, 24 mm) with left postcentral and inferior occipital gyri (positive) and with left parahippocampal gyrus (negative).
Figure 5.
Statistical significance of the decreases in the strength of the functional connectivity for seed regions that demonstrated lower FCD for the TSFC scans than for the RSFC scans (Tables 1 and 2; bold region labels).
Correlations with Ball-Tracking Accuracy
Increased BOLD-fMRI deactivation responses in ventral precuneus were linearly correlated with increased accuracy during the ball-tracking task (Fig. 6). During the TSFC scan, ball-tracking accuracy was negatively correlated with lFCD in visual cortex and postcentral gyrus, regions that showed lower lFCD for TSFC than for RSFC, such that improved accuracy was associated with lower lFCD (Table 3). The anterior thalamus, a region that showed moderated task-related lFCD decreases, also showed negative correlation between lFCD and accuracy during TSFC (Table 3). Ball-tracking accuracy was also negatively correlated with gFCD in both the visual cortex and the parietal regions (Table 3). The weak negative correlation between accuracy and fALFF in the visual cortex did not reach statistical significance (PFWE = 0.18). Ball-tracking accuracy was not correlated with ALFF in any brain region.
Figure 6.
Statistical t-score maps for the negative correlations with performance accuracy during blocked (BOLD fMRI; left column) and continuous (lFCD and gFCD; middle and right columns, respectively) ball tracking, superimposed on the surface of the Colin human brain template, and scatter plots exemplifying these correlations for the main clusters listed in Table 3. Blue circles mark the approximated locations of the clusters on the brain surface. Red lines are linear regression plots of the data. Labels indicate brain regions and Pearson correlation factors (R). Independent simple regression analyses for each image modality. Sample size: 45 healthy subjects.
Discussion
Here, we studied the functional connectivity for rest and task conditions using whole-brain data-driven graph theory measures and independent scanning for the continuous ball-tracking and resting-state conditions. The FCD in visual, auditory, language, somatosensory, and motor/premotor cortices was significantly lower during ball tracking than during the resting-state and showed significant negative correlations with performance accuracy, such that connectivity decreases in regions with weak activation during ball tracking were associated with better performance.
Most of the previous studies on the effect of task performance on functional integration were based on effective connectivity (Friston 2011), which is radically different to functional connectivity as it depends on a mathematical model, describing “how” areas are connected and a neuroanatomical model describing “which” areas are connected. Thus, effective connectivity is a powerful “hypothesis-driven” method for measuring the influence that one activated area exerts over another activated area (Friston et al. 1993). However, whereas effective connectivity is ideal to study the functional integration of the regions engaged by a given task, it cannot access the functional integration of brain regions not engaged by the task.
Other studies that recently evaluated the effect of task performance on functional connectivity used the psychophysiological interaction and independent component analyses (Sambataro et al. 2013; Ezekiel et al. 2012), or contrasted RSFC measures collected prior and after the performance of a cognitive fMRI task (Gordon et al. 2012). These and other similar approaches have been used in the past to assess changes in functional connectivity between rest and task conditions (Arfanakis et al. 2000; Lowe et al. 2000; Hampson et al. 2002, 2004; Jiang et al. 2004; Morgan and Price 2004; Bartels and Zeki 2005; Fransson 2006; Nir et al. 2006; Fair et al. 2007; Sun et al. 2007; Zhang and Li 2012). Here, we propose a different approach for accessing task-related changes in the functional integration that is based on graph theory metrics. Furthermore, we used correlation analyses to associate task-related FCD with brain activation during the performance of a visual attention task. The main finding of the study is that disconnection of task-irrelevant regions that are not captured by standard BOLD-fMRI activation/deactivation patterns significantly improved performance accuracy during ball tracking.
The task-related FCD decreases had a minimal spatial overlap with BOLD-fMRI activation patterns and involved brain regions that might interfere with spatial attention. Brain activation studies demonstrated that ball tracking predominantly engages areas of the dorsal stream that are also associated with the representation of object locations and motion, such as posterior and superior parietal, lateral occipital and dorsolateral prefrontal cortices, cerebellum, and thalamus. Furthermore, brain activation studies demonstrated that ball tracking disengages DMN regions, visual and auditory cortices, and insula (Tomasi et al. 2004, 2011; Tomasi, Wang, et al. 2009). Our previous work on this task identified specific visual attention processing in the SPC and the superior occipital gyrus (Tomasi, Chang, et al. 2007), regions that did not show task-related changes in FCD or linear associations between FCD and accuracy in the present study.
Task-related FCD decreases occurred in regions that had weak activation or significant deactivation (except for language areas and cerebellar vermis that showed moderate positive BOLD-fMRI activation and task-related decreases in FCD), suggesting that FCD decreases reflect the minimization of interfering neural processing in these regions. As a consequence, the patterns of brain activation and those of task-related FCD decreases were minimally overlapping (Fig. 4A), suggesting topographic differences between BOLD-fMRI responses and task-related FCD decreases. The pronounced negative correlation (R = −0.92; Fig. 4B) reflects the absence of FCD changes in activated regions and significant FCD decreases in deactivated or weakly activated regions. Therefore, the negative correlation between BOLD-fMRI activation and task-related FCD decreases across regions suggests the functional disconnection of task-irrelevant networks during task performance.
Competing neural processes such as those produced by attention to sensory stimuli could interfere with visual attention performance and be partially inhibited during ball tracking to enhance visual indexing efficiency (Tomasi et al. 2006). For instance, deactivation of auditory cortices, insula, and DMN areas has been associated with the need for focused attention (Lawrence et al. 2003; McKiernan et al. 2003) and improved accuracy (Tomasi et al. 2006). In the present study, increased DMN deactivation was also associated with improved accuracy during ball tracking (Fig. 6).
The proposed functional connectivity approach and BOLD fMRI seem to be complementary. Whereas BOLD fMRI highlights regions that were engaged during the visual attention task, task-related FCD differences highlight local and global hubs that show reduced connectivity to inhibit the interference from these regions in order to maximize accuracy during the ball-tracking task. Brain deactivation captures some of these hubs (ventral precuneus hub), but not other regions that were weakly activated during ball tracking. Strikingly, FCD hubs in the visual cortex as well as those in language (supramarginal and inferior frontal) and prefrontal association areas did not show significant BOLD-fMRI responses (activation or deactivation), but demonstrated pronounced lower connectivity during TSFC than during RSFC conditions. On the other hand, the primary motor cortex and insula showed pronounced BOLD-fMRI deactivation without significant task-related FCD decreases.
The similarity of the patterns suggests that task-related differences in FCD tend to occur in brain areas that are relevant for functional specialization (local connectivity) and functional integration (global connectivity) during resting conditions but less so during ball-tracking performance. It is well known that different visual cortex areas are specialized in different aspects of visual perception (DeYoe and Van Essen 1985; Shipp and Zeki 1985). For instance, there is evidence of functional integration in the primary visual area (V1) and between V1 and other visual areas, posterior parietal regions of the dorsal stream (“where” pathway), and temporal regions of the ventral stream (“what” pathway; Haxby et al. 1991). The seed-voxel correlation analyses demonstrated significant task-related connectivity decreases between the calcarine cortex and other visual areas that did not show significant BOLD-fMRI responses, and posterior parietal and temporal areas that showed BOLD-fMRI deactivation responses. This suggests lesser integration of V1 with other specialized visual areas, and with the dorsal and ventral streams during ball tracking than during resting conditions, which might reflect greater focus on the target balls and lesser focus on the more complex surrounding environment.
The ability to reduce the connectivity of the task-irrelevant hubs could be crucial in order to minimize interference during cognitive performance. Higher accuracy was associated with lower FCD in several regions that also showed task-related decreases in FCD (Fig. 6). The correlations with accuracy emphasize the importance of BOLD-fMRI deactivation in ventral precuneus, which is consistent with our previous studies (Tomasi et al. 2006) and could reflect minimization of introspection or self-referential thoughts during ball tracking. However, BOLD fMRI did not highlight the involvement of other brain regions that showed pronounced negative correlations between FCD and accuracy (V1 and V2 but also encompassed V3–V5, somatosensory motor and premotor cortices, superior and inferior parietal regions, and anterior thalamus). Interestingly, the correlations between performance and FCD were more extensive than those associated with BOLD activation (limited only to precuneus), which suggest that FCD may reflect better the complex patterns of network responses that are necessary for optimal performance. In this respect, our results suggests that task-related FCD modulations play crucial roles in brain function and that studies combining BOLD fMRI, and functional connectivity might offer a more complete picture of brain function and its disruption by disease.
Limitations
The continuous tracking condition of the TSFC scan was similar to the task epochs of the blocked ball-tracking task. However, the resting-state condition (eyes open; white fixation cross on a black background) was different from the resting baseline epochs of the ball-tracking task (subjects fixated their eyes on a black background with a central white cross and ignored the moving balls). This could enhance the disengagement during the RSFC and amplify the connectivity differences between RSFC and TSFC in the brain regions recruited during the resting baseline epochs of the blocked ball-tracking task (primary visual cortex). Differential head motion could confound functional connectivity differences between RSFC and TSFC. However, motion is unlikely to affect the FCD and ALFF measures in the present study, because motion measures (mean motion and FD) were not different for RSFC and TSFC scans. Motion correction techniques, such as “scrubbing”, could also confound functional connectivity differences between RSFC and TSFC. The scrubbing approach, however, is unlikely to originate the connectivity differences, as they were not significantly different for scrubbed and unscrubbed datasets. Lastly, there was a pronounced ceiling effect in performance accuracy data (Fig. 6) that resulted in a nonuniform distribution that limits the interpretation of the results.
Funding
This work was accomplished with support from the National Institutes of Alcohol Abuse and Alcoholism (2RO1AA09481).
Notes
Conflict of Interest: None declared.
References
- Arfanakis K, Cordes D, Haughton V, Moritz C, Quigley M, Meyerand M. Combining independent component analysis and correlation analysis to probe interregional connectivity in fMRI task activation datasets. Magn Reson Imaging. 2000;18:921–930. doi: 10.1016/S0730-725X(00)00190-9. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston K. Nonlinear spatial normalization using basis functions. Hum Brain Mapping. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4&lt;254::AID-HBM4&gt;3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A, Zeki S. Brain dynamics during natural viewing conditions—a new guide for mapping connectivity in vivo. Neuroimage. 2005;24:339–349. doi: 10.1016/j.neuroimage.2004.08.044. [DOI] [PubMed] [Google Scholar]
- Biswal B, Mennes M, Zuo X, Gohel S, Kelly C, Smith S, Beckmann C, Adelstein J, Buckner R, Colcombe S, et al. Toward discovery science of human brain function. Proc Natl Acad Sci. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caparelli EC, Tomasi D, Arnold S, Chang L, Ernst T. k-Space based summary motion detection for functional magnetic resonance imaging. Neuroimage. 2003;20:1411–1418. doi: 10.1016/S1053-8119(03)00339-2. [DOI] [PubMed] [Google Scholar]
- Chang L, Tomasi D, Yakupov R, Lozar C, Arnold S, Caparelli E, Ernst T. Adaptation of the attention network in human immunodeficiency virus brain injury. Ann Neurol. 2004;56:259–272. doi: 10.1002/ana.20190. [DOI] [PubMed] [Google Scholar]
- DeYoe E, Van Essen D. Segregation of efferent connections and receptive field properties in visual area V2 of the macaque. Nature. 1985;317:58–61. doi: 10.1038/317058a0. [DOI] [PubMed] [Google Scholar]
- Ezekiel F, Bosma R, Morton J. Dimensional change card sort performance associated with age-related differences in functional connectivity of lateral prefrontal cortex. Dev Cogn Neurosci. 2012;5C:40–50. doi: 10.1016/j.dcn.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D, Schlaggar B, Cohen A, Miezin F, Dosenbach N, Wenger K, Fox M, Snyder A, Raichle M, Petersen S. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Harrison B, Zalesky A, Simons J. Competitive and cooperative dynamics of large-scale brain functional networks supporting recollection. Proc Natl Acad Sci USA. 2012;109:12788–12793. doi: 10.1073/pnas.1204185109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M, Raichle M. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox M, Snyder A, Vincent J, Corbetta M, Van Essen D, Raichle M. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M, Snyder A, Zacks J, Raichle M. Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nat Neurosci. 2006;9:23–25. doi: 10.1038/nn1616. [DOI] [PubMed] [Google Scholar]
- Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44:2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Friston K. Functional and effective connectivity: a review. Brain Connect. 2011;1:13–36. doi: 10.1089/brain.2011.0008. [DOI] [PubMed] [Google Scholar]
- Friston K, Frith C, Frackowiak R. Time-dependent changes in effective connectivity measured with PET. Hum Brain Mapping. 1993;1:69–79. doi: 10.1002/hbm.460010108. [DOI] [Google Scholar]
- Gordon E, Breeden A, Bean S, Vaidya C. Working memory-related changes in functional connectivity persist beyond task disengagement. Hum Brain Mapping. 2012 doi: 10.1002/hbm.22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigg O, Grady C. Task-related effects on the temporal and spatial dynamics of resting-state functional connectivity in the default network. Plos One. 2010;5:e13311. doi: 10.1371/journal.pone.0013311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Olson I, Leung H, Skudlarski P, Gore J. Changes in functional connectivity of human MT/V5 with visual motion input. Neuroreport. 2004;15:1315–1319. doi: 10.1097/01.wnr.0000129997.95055.15. [DOI] [PubMed] [Google Scholar]
- Hampson M, Peterson B, Skudlarski P, Gatenby J, Gore J. Detection of functional connectivity using temporal correlations in MR images. Hum Brain Mapping. 2002;15:247–262. doi: 10.1002/hbm.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby J, Grady C, Horwitz B, Ungerleider L, Mishkin M, Carson R, Herscovitch P, Schapiro M, Rapoport S. Dissociation of object and spatial visual processing pathways in human extrastriate cortex. Proc Natl Acad Sci USA. 1991;88:1621–1625. doi: 10.1073/pnas.88.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig J, Scheffler K. Hyperechoes. Magn Reson Med. 2001;46:6–12. doi: 10.1002/mrm.1153. [DOI] [PubMed] [Google Scholar]
- Jiang T, He Y, Zang Y, Weng X. Modulation of functional connectivity during the resting state and the motor task. Hum Brain Mapping. 2004;22:63–71. doi: 10.1002/hbm.20012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannurpatti S, Rypma B, Biswal B. Prediction of task-related BOLD fMRI with amplitude signatures of resting-state fMRI. Front Syst Neurosci. 2012;6:7. doi: 10.3389/fnsys.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence N, Ross T, Hoffmann R, Garavan H, Stein E. Multiple neuronal networks mediate sustained attention. J Cogn Neurosci. 2003;15:1028–1038. doi: 10.1162/089892903770007416. [DOI] [PubMed] [Google Scholar]
- Lee JH, Garwood M, Menon R, Adriany G, Andersen P, Truwit CL, Ugurbil K. High contrast and fast three-dimensional magnetic resonance imaging at high fields. Magn Reson Med. 1995;34:308–312. doi: 10.1002/mrm.1910340305. [DOI] [PubMed] [Google Scholar]
- Lowe M, Dzemidzic M, Lurito J, Mathews V, Phillips M. Correlations in low-frequency BOLD fluctuations reflect cortico-cortical connections. Neuroimage. 2000;12:582–587. doi: 10.1006/nimg.2000.0654. [DOI] [PubMed] [Google Scholar]
- McKiernan K, Kaufman J, Kucera-Thompson J, Binder J. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Mennes M, Kelly C, Zuo X, Di Martino A, Biswal B, Castellanos F, Milham M. Inter-individual differences in resting state functional connectivity predict task-induced BOLD activity. Neuroimage. 2010;50:1690–1701. doi: 10.1016/j.neuroimage.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan V, Price R. The effect of sensorimotor activation on functional connectivity mapping with MRI. Magn Reson Imaging. 2004;22:1069–1075. doi: 10.1016/j.mri.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Nir Y, Hasson U, Levy I, Yeshurun Y, Malach R. Widespread functional connectivity and fMRI fluctuations in human visual cortex in the absence of visual stimulation. Neuroimage. 2006;30:1313–1324. doi: 10.1016/j.neuroimage.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Power J, Barnes K, Snyder A, Schlaggar B, Petersen S. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pylyshyn Z. The role of location indexes in spatial perception: a sketch of the FINST spatial-index model. Cognition. 1989;32:65–97. doi: 10.1016/0010-0277(89)90014-0. [DOI] [PubMed] [Google Scholar]
- Pylyshyn ZW, Storm RW. Tracking multiple independent targets: evidence for a parallel tracking mechanism. Spatial Vis. 1988;3:179–197. doi: 10.1163/156856888X00122. [DOI] [PubMed] [Google Scholar]
- Raichle M. Two views of brain function. Trends Neurosci. 2010;14:180–190. doi: 10.1016/j.tics.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Sambataro F, Mattay V, Thurin K, Safrin M, Rasetti R, Blasi G, Callicott J, Weinberger D. Altered cerebral response during cognitive control: a potential indicator of genetic liability for schizophrenia. Neuropsychopharmacology. 2013;38:846–853. doi: 10.1038/npp.2012.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears C, Pylyshyn Z. Multiple object tracking and attentional processing. Can J Exp Psychol. 2000;54:1–14. doi: 10.1037/h0087326. [DOI] [PubMed] [Google Scholar]
- Shipp S, Zeki S. Segregation of pathways leading from area V2 to areas V4 and V5 of macaque monkey visual cortex. Nature. 1985;315:322–325. doi: 10.1038/315322a0. [DOI] [PubMed] [Google Scholar]
- Smith S, Fox P, Miller K, Glahn D, Fox P, Mackay C, Filippini N, Watkins K, Toro R, Laird A, et al. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci USA. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, Miller K, Moeller S, Xu J, Auerbach E, Woolrich M, Beckmann C, Jenkinson M, Andersson J, Glasser M, et al. Temporally-independent functional modes of spontaneous brain activity. Proc Natl Acad Sci. 2012;109:3131–3136. doi: 10.1073/pnas.1121329109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Miller L, Rao A, D'Esposito M. Functional connectivity of cortical networks involved in bimanual motor sequence learning. Cereb Cortex. 2007;17:1227–1234. doi: 10.1093/cercor/bhl033. [DOI] [PubMed] [Google Scholar]
- Tian L, Ren J, Zang Y. Regional homogeneity of resting state fMRI signals predicts Stop signal task performance. Neuroimage. 2012;60:539–544. doi: 10.1016/j.neuroimage.2011.11.098. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Caparelli EC, Chang L, Ernst T. fMRI-acoustic noise alters brain activation during working memory tasks. Neuroimage. 2005;27:377–386. doi: 10.1016/j.neuroimage.2005.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Chang L, Caparelli E, Ernst T. Different activation patterns for working memory load and visual attention load. Brain Res. 2007;1132:158–165. doi: 10.1016/j.brainres.2006.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Ernst T, Caparelli E, Chang L. Common deactivation patterns during working memory and visual attention tasks: an intra-subject fMRI study at 4 Tesla. Hum Brain Mapping. 2006;27:694–705. doi: 10.1002/hbm.20211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Ernst T, Caparelli EC, Chang L. Practice-induced changes of brain function during visual attention: a parametric fMRI study at 4 Tesla. Neuroimage. 2004;23:1414–1421. doi: 10.1016/j.neuroimage.2004.07.065. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Goldstein R, Telang F, Maloney T, Alia-Klein N, Caparelli E, Volkow N. Thalamo-cortical dysfunction in cocaine abusers: implications in attention and perception. Psych Res Neuroimaging. 2007;155:189–201. doi: 10.1016/j.pscychresns.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow N. Functional connectivity density mapping. Proc Natl Acad Sci USA. 2010;107:9885–9890. doi: 10.1073/pnas.1001414107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow N. Functional connectivity hubs in the human brain. Neuroimage. 2011;57:908–917. doi: 10.1016/j.neuroimage.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow N, Wang R, Telang F, Wang G, Chang L, Ernst T, Fowler J. Dopamine transporters in striatum correlate with deactivation in the default mode network during visuospatial attention. PLoS ONE. 2009;4:e6102. doi: 10.1371/journal.pone.0006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow N, Wang G, Wang R, Telang F, Caparelli E, Wong C, Jayne M, Fowler J. Methylphenidate enhances brain activation and deactivation responses to visual attention and working memory tasks in healthy controls. Neuroimage. 2011;54:3101–3110. doi: 10.1016/j.neuroimage.2010.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Wang R, Telang F, Boronikolas V, Jayne M, Wang G, Fowler J, Volkow N. Impairment of Attentional networks after 1 night of sleep deprivation. Cerebr Cortex. 2009;19:233–240. doi: 10.1093/cercor/bhn073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M, Stam C, Boersma M, Hulshoff Pol H. Small-world and scale-free organization of voxel-based resting-state functional connectivity in the human brain. Neuroimage. 2008;43:528–539. doi: 10.1016/j.neuroimage.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Van Dijk K, Sabuncu M, Buckner R. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Liu J, Zhong N, Qin Y, Zhou H, Li K. Changes in the brain intrinsic organization in both on-task state and post-task resting state. Neuroimage. 2012;62:394–407. doi: 10.1016/j.neuroimage.2012.04.051. [DOI] [PubMed] [Google Scholar]
- Wig G, Buckner R, Schacter D. Repetition priming influences distinct brain systems: evidence from task-evoked data and resting-state correlations. J Neurophysiol. 2009;101:2632–2648. doi: 10.1152/jn.91213.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Long X, Yang Y, Yan H, Zhu C, Zhou X, Zang Y, Gong Q. Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI. Neuroimage. 2007;36:144–152. doi: 10.1016/j.neuroimage.2007.01.054. [DOI] [PubMed] [Google Scholar]
- Yantis S, Johnston J. On the locus of visual selection: evidence from focused attention tasks. J Exp Psychol Hum Percept Perform. 1990;16:135–149. doi: 10.1037/0096-1523.16.1.135. [DOI] [PubMed] [Google Scholar]
- Zhang S, Li C. Task-related, low-frequency task-residual, and resting state activity in the default mode network brain regions. Front Psychol. 2012;3:172. doi: 10.3389/fpsyg.2012.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Q, Zhu C, Yang Y, Zuo X, Long X, Cao Q, Wang Y, Zang Y. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods. 2008;172:137–141. doi: 10.1016/j.jneumeth.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]