Abstract
The ESR radiation dosimetric properties of tooth enamel samples from cows and goats were investigated and compared with those of human samples. Samples were prepared first mechanically, and then chemically. The study results showed that the native signals from cow and goat samples were weaker than those from human samples; the radiation sensitivities for cow and goat samples were very close to those of human tooth enamel samples. These results indicated that cow and goat teeth could be alternative materials for radiation dose estimation.
Keywords: animal tooth enamel, ESR, EPR, dosimetry
INTRODUCTION
Radiation-induced paramagnetic carbon dioxide (CO2−) radicals are generated from the carbonate ions in human tooth enamel. They are stable and show a linear radiation response over a large dose range [1–3]. Due to its high sensitivity, electron spin resonance (ESR) spectroscopy of human tooth enamel has been broadly used for dose reconstruction for victims of a number of nuclear and radiation accidents, as well as that associated with medical X-ray diagnostics [4–7]. Although in vivo ESR tooth dosimetry is developing rapidly and is used to examine survivors of accidents involving radiation during triage [8, 9], in vivo measurements currently have a standard error of 1 Gy, doses lower than ∼2 Gy are undetectable [10], and thus this is not a suitable technique for evaluating low-dose exposures.
ESR dosimetry is used to determine accurate doses in vitro as a result of a radiation accident, but because extracted human teeth are not always available, and collecting teeth from irradiated persons may raise ethical concerns, animal teeth may be used to estimate the radiation dose. Previous research on animal teeth ESR dosimetry [11–14] has demonstrated that teeth from a number of animals can be used as alternatives when human teeth are difficult to obtain. Toyoda et al. indicate that the sensitivity of the radiation-induced signal from cow tooth enamel varies significantly from sample to sample [13], so it is necessary to collect samples from a number of areas.
In Inner Mongolia and some areas of northern China, cows and goats are the main domestic animals. They are raised for food supply and commercial purposes by nomadic people and are living very closely with human beings. The radiation dose received by these animals should be at the same level as that of the humans around them, so it is important to study the ESR dosimetric properties in detail.
Cows and goats are herbivores with high crown molars; the crown of the tooth is extremely long compared to that of a human molar, enamel covers the crown, and enamel is also located inside the tooth. The top surface of the crown will gradually wear away as a result of grinding plant materials, but the enamel on the lateral surface and inside the tooth will persist and thus be suitable for ESR dosimetry.
Every free radical species has a unique g-factor that is dependent on both the spin and orbital angular momenta of the unpaired electron [7]. After irradiation, the ESR signal of the tooth enamel consists of two main signals. One is the radiation-induced dosimetric signal (DS) with perpendicular and parallel g-factor components of 2.0018 and 1.9975, respectively; the peak-to-peak amplitude of the perpendicular part is used for dose reconstruction. The other ESR signal is comprised of the native signal (NS), independent of the radiation, which is characterized by g-factor of 2.0046. Because of individual differences in the shape of the NS, the separation of the NS and DS will lead to a variation in the dose assessment at low radiation doses [15]. Therefore, the shape of the NS is as important as the dose response. In this study, NS shapes and the dose response from cow and goat samples were investigated and compared with those of human tooth enamel samples.
MATERIALS AND METHODS
Subjects
In this study, tooth enamel of cows, goats and humans was examined. Animal teeth were collected at a ranch on prairie in Inner Mongolia, where little environmental radioactive contamination was found. The teeth were collected from five cows aged 1, 2, 3, 5 and 7 years respectively, and, similarly, teeth from five goats aged 1, 2, 3, 5 and 6 years were obtained. The ages of these tooth samples were confirmed by a group of professional veterinarians. As the front teeth of the animals are likely to have been exposed to sunlight [16, 17], only molar teeth were used in this study. Nine human molar teeth were supplied by adults aged 18–54 (extracted by dentists in Nantong city for medical reasons).
Methods
Methods of ESR dosimetry were established for teeth of cows and goats, and involved animal teeth collection, enamel sample preparation, and assessment of radiation dose responses. The roots of cow and goat molars were cut off with a saw. Every tooth was cut into pieces, and then dentin was removed using a dental drill. Because of the complicated structure of cow and goat enamel, in order to remove the dentin completely, the teeth were treated with 20% NaOH aqueous solution, as described by Toyoda et al. [18]. Every 3 d, the samples were taken out of the solution and the softened remaining dentin was removed with a knife. After the procedures had been repeated three times and the dentin was completely removed, the tooth enamel was washed with distilled water in an ultrasonic bath at 60°C for 8 h. After drying at room temperature, the tooth enamel was crushed to a 0.5–1.0-mm grain size using a nipper; the enamel collected from each animal was mixed, and five enamel samples were prepared from five cows and labeled C-1 to C-5. Enamel from five goats (labeled G-1 to G-5) was obtained in a similar fashion. Five aliquots of 100 mg from C-1 to C-5 and from G-1 to G-5 were taken for dose–response assessment.
After the roots of nine human teeth (labeled H-1 to H-9) were cut off and the tooth dentin was removed using a dental drill, the teeth were soaked in 30% NaOH aqueous solution for 1 week. They were then washed with distilled water before drying at room temperature, and the enamel chips were then crushed into 0.5–1.0 mm particles. Five aliquots of 100 mg were taken from each human tooth for ESR spectra registration. Research results of Haskell et al. [19] and Toyoda et al. [13] indicate that mechanical and chemical treatments do not affect the retrospective doses of human or cow teeth.
Samples were irradiated by a 137Cs gamma ray source at the Institute of Radiation Medicine, at the Chinese Academy of Medical Sciences. Radiation doses were 0, 0.5, 1, 2 and 5 Gy for each type of samples and this was delivered at a dose rate of 12.5 mGy/s.
ESR spectra measurements
ESR spectra measurements were performed at room temperature with a Bruker A-300 spectrometer. We obtained optimal experimental parameters in the pre-experiment. The main spectral recording parameters were: 2 mW microwave power, 0.3 mT modulation amplitude, 100 kHz modulation frequency, 10 mT sweep width, 163 ms receiver time constant, 166.9 s sweep time, number of accumulations 10, and 1024 channel resolution.
Before the measurement of samples each day, an ER 4119HS-2100 marker (g = 1.9800 with one 3G single line) was scanned for g factor calibration, which was also used as an amplitude reference to correct for spectrometer fluctuations. The ER 4119HS high-sensitivity cavity is a multi-purpose X-band cavity with cooling site plates, and its unloaded Q-value is 6000. Its spectrum was recorded under the same conditions as the spectral measurements, which enables us to determine the small shift in magnetic field between scans and to check the amplitude in order to correct the sample spectra.
Before- and after-radiation ESR spectra of all samples were measured in three directions for the existence of anisotropy. We measured once in each direction. The parameters, peak-to-peak amplitude and line width of the NSs were obtained from measurements of the unirradiated samples. The amplitudes of the DSs were obtained from spectra-fitting processing as described by Wu et al. [6]. The slope of the linear regression of DS indicates the radiation response.
Statistical analysis
For repeated measurements, we used the mean value as the measurement result and the standard deviation of the mean value as the experimental uncertainty UA. In this study, UA was mainly caused by data from ESR spectra measurements and analysis, and was calculated using the following formula:
| (1) |
where S(x) is the standard deviation of one group of samples.
S(x) is a function, which is the standard deviation divided by the square root of the number of measurements. Here X is a parameter. Other possible sources, such as sample preparation, ESR measurements and numerical treatment of spectra, may contribute to the uncertainty of tooth enamel ESR, and may result in an uncertainty (UB) of 15% [7, 19]. The overall combined uncertainties were obtained using formula (2):
| (2) |
According to the ISO Guide [20] for radiation measurements, one can assume that taking K = 2 produces an interval having a level of confidence of ∼95%.
RESULTS
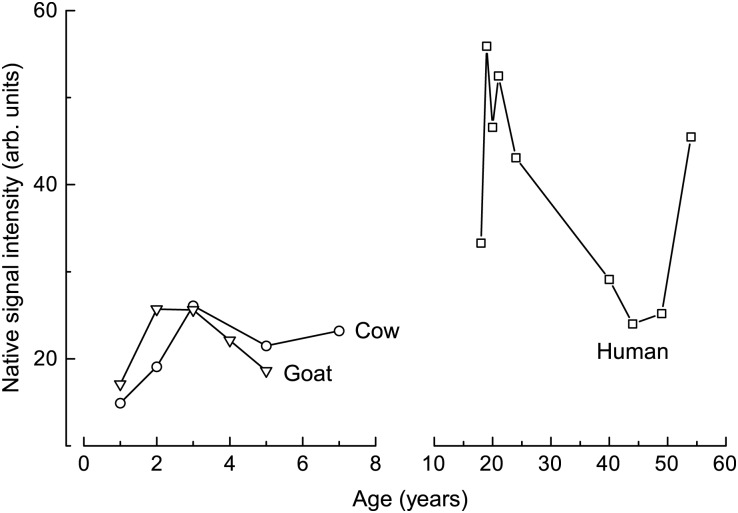
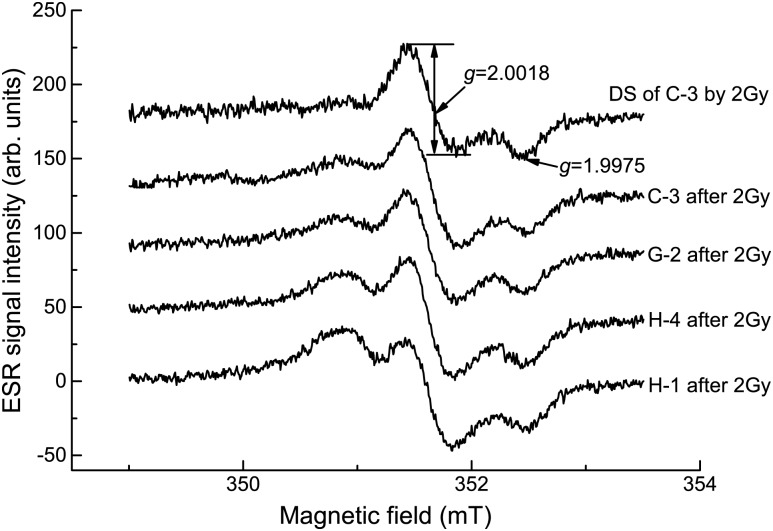
The NSs (with a g-factor of 2.0046) of human, cow and goat enamel samples were measured, and some typical spectra of two people (H-1 and H-4), a 3-year-old cow (C-3) and a 2-year-old goat (G-2) are given in Fig. 1. The results of the peak-to-peak line width and peak-to-peak amplitude for NS are shown in Fig. 2 and Table 1. Radiation sensitivity (as shown in Table 1) is determined from the uncertainty of the regression line slope in the dose dependence of the DS amplitude for each sample.
Fig. 1.
Native ESR spectra of different tooth enamel samples.
Fig. 2.
Native signal intensities of different aged samples.
Table 1.
The parameters of ESR spectral shape and radiation dose sensitivity for three species of tooth enamel samples.
| Species | Sample number | Ages (years) | Native signal |
Dosimetric signal |
|
|---|---|---|---|---|---|
| ΔH (mT) | Amplitude (arb.) units) | Radiation sensitivity (Gy−1) | |||
| Human | H-1 | 19 | 0.74 ± 0.02 | 55.9 ± 2.9 | 33.8 ± 1.0 |
| H-2 | 21 | 0.71 ± 0.03 | 52.5 ± 3.0 | 35.3 ± 3.3 | |
| H-3 | 20 | 0.77 ± 0.01 | 46.6 ± 1.8 | 36.0 ± 1.2 | |
| H-4 | 18 | 0.76 ± 0.02 | 33.3 ± 1.4 | 38.6 ± 0.5 | |
| H-5 | 24 | 0.74 ± 0.02 | 43.1 ± 2.6 | 32.7 ± 1.2 | |
| H-6 | 54 | 0.71 ± 0.01 | 45.5 ± 2.9 | 36.2 ± 1.5 | |
| H-7 | 40 | 0.76 ± 0.03 | 29.1 ± 2.1 | 33.8 ± 0.6 | |
| H-8 | 49 | 0.74 ± 0.01 | 25.2 ± 2.1 | 41.6 ± 0.4 | |
| H-9 | 44 | 0.73 ± 0.02 | 24.0 ± 1.5 | 39.0 ± 0.3 | |
| Average for human teetha | 0.74 ± 0.02 | 39.5 ± 11.9 | 36.3 ± 2.9 | ||
| U at 95% confidence level | 0.22 | 14.2 ± 1.0 | 11.1 | ||
| Cow | C-1 | 1 | 0.70 ± 0.03 | 14.9 ± 0.9 | 32.1 ± 0.4 |
| C-2 | 2 | 0.87 ± 0.02 | 19.1 ± 1.0 | 32.6 ± 0.6 | |
| C-3 | 3 | 0.86 ± 0.02 | 26.1 ± 1.3 | 34.5 ± 0.5 | |
| C-4 | 5 | 0.78 ± 0.03 | 21.5 ± 1.2 | 35.4 ± 0.4 | |
| C-5 | 7 | 0.78 ± 0.02 | 23.2 ± 1.3 | 37.1 ± 0.8 | |
| Average for cow teeth | 0.80 ± 0.07 | 21.0 ± 4.2 | 34.4 ± 2.0 | ||
| U at 95% confidence level | 0.25 | 7.3 | 10.5 | ||
| Goat | G-1 | 1 | 0.68 ± 0.03 | 17.1 ± 1.1 | 33.5 ± 0.6 |
| G-2 | 2 | 0.73 ± 0.02 | 25.7 ± 1.4 | 35.6 ± 0.6 | |
| G-3 | 3 | 0.73 ± 0.01 | 25.6 ± 1.1 | 36.9 ± 0.7 | |
| G-4 | 5 | 0.73 ± 0.02 | 22.1 ± 1.2 | 33.3 ± 0.8 | |
| G-5 | 6 | 0.76 ± 0.03 | 18.6 ± 1.1 | 32.2 ± 0.5 | |
| Average for goat teeth | 0.73 ± 0.03 | 21.5 ± 4.0 | 34.3 ± 1.9 | ||
| U at 95% confidence level | 0.22 | 7.4 | 10.4 | ||
aAverage value = Mean ± STD.
The ESR spectra after 2-Gy irradiation of C-3, G-2, H-4 and H-1 are shown in Fig. 3, and the upper line is the DS spectrum of the 2-Gy-exposed C-3 sample (obtained by subtraction of the simulated NS spectrum from the ESR spectrum after 2 Gy irradiation). The DS intensity of the H-1 sample was affected more than other samples, which had smaller NS values.
Fig. 3.
ESR spectra of different tooth enamel samples irradiated with a gamma ray dose of 2 Gy.
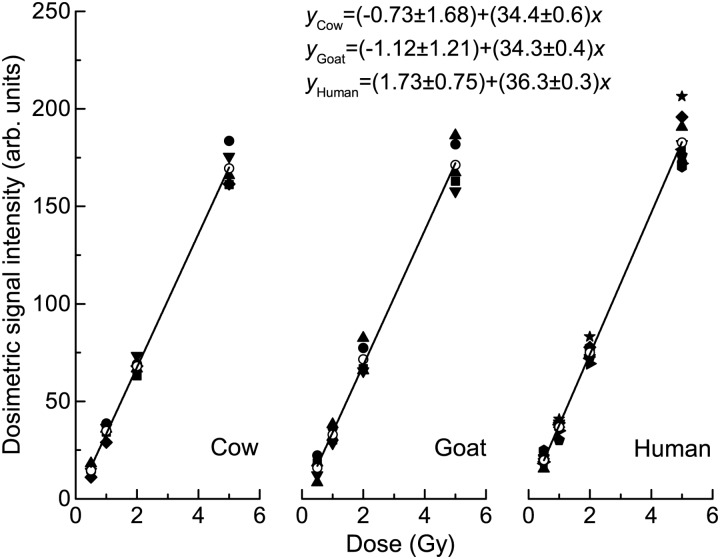
The intensities of DS versus gamma ray radiation from nine human, five cow and five goat samples are displayed in Fig. 4 and Table 1. Dependences of the intensities on gamma ray radiation were processed using the linear regression method, in which the slope of the linear regression line indicates the gamma ray radiation response per Gy for the DS of a 100-mg sample. The linear regression lines of the average intensities, their fitting lines and linear regression equations are also presented.
Fig. 4.
Radiation dose responses of cow, goat and human samples. Open circles indicate the average responses.
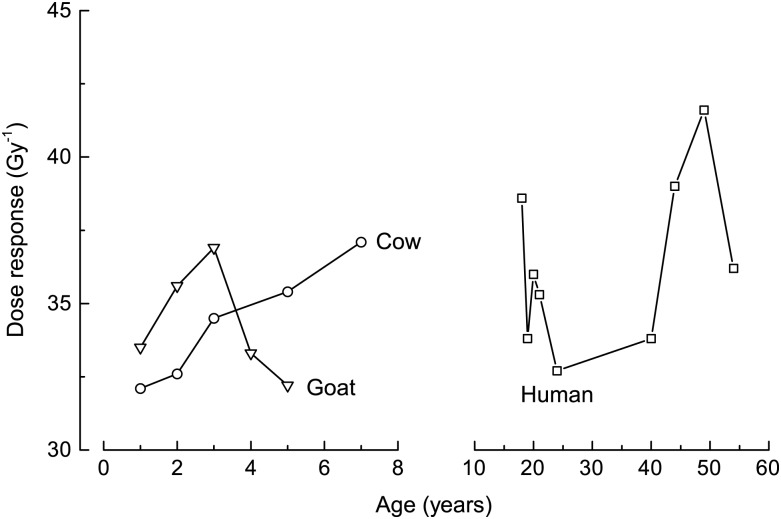
Dose response versus sample ages for human, cow and goat samples are presented in Fig. 5 and Table 1. For the three kinds of samples, there were no significant correlations between DS dose response, NS shape or sample ages.
Fig. 5.
Radiation sensitivities of different aged cow, goat and human samples.
DISCUSSION
The intensities of the NS for the human samples varied from 24.0–55.9 (arb. units), and the 95% confidence interval was (25.2, 53.7) (arb. units). The intensity measured for H-1 was more than twice that of either H-8 or H-9. For the cow samples, the NS intensity varied from 14.9–26.1(arb. units), and the 95% confidence interval was (13.7, 28.3) (arb. units). For the goat samples, the NS intensity varied from 17.1–25.7 (arb. units), and the 95% confidence interval was (14.1, 28.9) (arb. units). It can be observed that the intensities of the NS for cows and goats were less than those of human samples.
The average NS intensities of the cow and goat teeth were ∼21.0 (arb. units), i.e. similar to the lower values of the human samples. The analysis of variance (ANOVA) result indicated that the NS intensities for the cow and goat samples were not significantly different (P = 0.87), but that the NS intensities for the human samples were significantly different from those of the cow and goat samples (P = 0.002; considered significant when P < 0.05). Possible reasons for this effect are the different mechanical and chemical preparation procedures and race variation. Also, the higher NS level and the variation in the human samples were likely a result of dental disease because of unhealthy hygiene and dietary habits or individual differences in diet. The larger range in the age of the human teeth (from 18–54 years) as compared with that of the animals (from 1–7 years) may also have contributed to the greater range in the NS results for the human samples. No correlation between NS shapes and sample ages is indicated in Fig. 2.
We can see that the line width of NSs for human samples ranged from 0.71–0.77 mT. The NS line width of the cow teeth varied from 0.70–0.87mT, and that of the goat samples varied from 0.68 to 0.76 mT. From the analysis of variance (ANOVA) result, we determined that the NS intensities for the cow and goat samples were not significantly different (P = 0.87), whereas the NS intensities for the human samples was significantly different from those for cow and goat (P = 0.002; considered significant when P < 0.05).
The results mentioned above indicate the variation in the NS intensity and line width for the various samples. Since only five cow teeth and five goat teeth were examined, more samples of animals encompassing a greater variety of genetic material should be collected for further study.
The radiation dose responses of nine human samples ranged from 32.7–41.6 Gy−1, and the 95% confidence interval was (25.2, 47.4) Gy−1. For cows of different ages, the radiation sensitivity varied from 32.1–37.1 Gy−1, and the 95% confidence interval was (23.9, 47.4) Gy−1. For goats of different ages, the radiation sensitivity varied from 32.2–36.9 Gy−1, and the 95% confidence interval was (23.9, 44.7) Gy−1. Comparing the radiation responses of the three kinds of enamel samples, we found that the averaged responses of the cows and goats were similar to those of the human samples, and the ANOVA result showed that the radiation dose response for human, cow and goat samples were not significantly different from one another. Toyoda et al. [13] reported a more varied dose response of cow teeth from the South Ural region; this result may be due to the environmental pollution and the diet in the Ural region. Another possible reason is the difference in sample preparation technique. Further studies are necessary, with cow and goat teeth collected from different areas, in order to verify these results.
From the data given in Fig. 2 and Fig. 5, the dose responses for human enamel samples (four people, with ages ranging from 19 to 24, and one person with an age of 54) with higher NS intensities were relatively lower. Reasons for this effect could be individual variation, or extent and type of dental disease caused by some dietary and hygiene habits. Thus, it will be necessary to obtain a larger number of samples for further investigation in order to clarify this.
Figures 2 and 5 reveal that the dose responses of the cow and goat samples were close to those of the human samples, which indicates that these animal teeth are likely to be good alternatives to human teeth for dose reconstruction.
In summary, tooth enamel samples were successfully collected from cow and goat teeth using mechanical and chemical treatments. The NS intensities from the cow and goat samples were weaker than those from the human samples. The DS intensities for the three kinds of samples increased linearly with gamma ray dose. The dose responses for cow and goat samples were very close to those of human tooth samples. Since the usual lifetime of cows and goats is not more than 10 years, the cow and goat teeth can be used for dose reconstruction of recent radiation accidents when human teeth are not available. Also, it will be necessary to collect a large number of samples from a range of areas in order to investigate the spectral properties further for accurate dose evaluation.
FUNDING
This work was supported by funds from the Institute of Radiation Medicine, Chinese Academy of Medical Sciences (SR0816) and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (SRF for ROCS, SEM). Funding to pay the Open Access publication charges for this article was provided by the Institute of Radiation Medicine, Chinese Academy of Medical Sciences (Grant Number: SR0816) and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry.
REFERENCES
- 1.Wieser A. Review of reconstruction of radiation incident air kerma by measurement of absorbed dose in tooth enamel with EPR. Radiat Prot Dosimetry. 2012;149:71–8. doi: 10.1093/rpd/ncr446. [DOI] [PubMed] [Google Scholar]
- 2.Fattibene P, Callens F. EPR dosimetry with tooth enamel: a review. Appl Radiat Isot. 2010;68:2033–116. doi: 10.1016/j.apradiso.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 3.Fattibene P, Wieser A, Adolfsson E, et al. The 4th international comparison on EPR dosimetry with tooth enamel. Part 1: Report on the results. Radiat Meas. 2011;46:765–71. [Google Scholar]
- 4.Volchkova A, Shishkina EA, Ivanov D, et al. Harmonization of dosimetric information obtained by different EPR methods: Experience of the Techa river study. Radiat Meas. 2011;46:801–7. [Google Scholar]
- 5.Wieser A, Vasilenko E, Zankl M, et al. Evaluation of dose to tooth enamel from medical diagnostic X-ray examinations at Mayak PA. Radiat Meas. 2011;46:808–12. [Google Scholar]
- 6.Weizhang W, Ao Y, Wenyi Z, et al. Dose estimation by EPR spectroscopy of tooth enamel in Chinese medical diagnostic X-ray workers. Radiat Prot Dosimetry. 2006;118:102–5. doi: 10.1093/rpd/nci336. [DOI] [PubMed] [Google Scholar]
- 7.IAEA Report. Use of electron paramagnetic resonance dosimetry with tooth enamel for retrospective dose assessment. Report of a coordinated research project. IAEA-TecDoc-13312002.
- 8.Zdravkova M, Gallez B, Debuyst R. A comparative in vivo and in vitro L-band EPR study of irradiated rat incisors. Radiat Meas. 2005;39:143–8. [Google Scholar]
- 9.Demidenko E, Williams BB, Sucheta A, et al. Radiation dose reconstruction from L-band in vivo EPR spectroscopy of intact teeth: comparison of methods. Radiat Meas. 2007;42:1089–93. doi: 10.1016/j.radmeas.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams BB, Dong R, Flood AB, et al. A deployable in vivo EPR tooth dosimeter for triage after a radiation event involving large populations. Radiat Meas. 2011;46:772–7. doi: 10.1016/j.radmeas.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serezhenkov VA, Moroz IA, Klevezal GA, et al. Estimation of accumulated dose of radiation by method of ESR-spectrometry of dental enamel of mammals. Appl Radiat Isot. 1996;47:1321–8. doi: 10.1016/s0969-8043(96)00199-6. [DOI] [PubMed] [Google Scholar]
- 12.Toyoda S, Tanizawa H, Romanyukha AA, et al. Gamma-ray dose response of ESR signals in tooth enamel of cows and mice in comparison with human teeth. Radiat Meas. 2003;37:341–6. [Google Scholar]
- 13.Toyoda S, Romanyukha A, Hino Y, et al. Effect of chemical treatment on ESR dosimetry of cow teeth: application to the samples from Southern Urals. Radiat Meas. 2007;42:1178–80. [Google Scholar]
- 14.Hassan GM, Aboelezz E, El-Khodary A, et al. Inter-comparison study between human and cow teeth enamel for low dose measurement using ESR. Nucl Instrum Methods Phys Res B. 2010;268:2329–36. [Google Scholar]
- 15.Ivannikov AI, Tikunov DD, Skvortsov VG, et al. Elimination of the background signal in tooth enamel samples for EPR-dosimetry by means of physical–chemical treatment. Appl Radiat Isot. 2001;55:701–5. doi: 10.1016/s0969-8043(01)00116-6. [DOI] [PubMed] [Google Scholar]
- 16.Jiao L, Takada J, Endo S, et al. Effects of sunlight exposure on the human tooth enamel ESR spectra used for dose reconstruction. J Radiat Res. 2007;48:21–9. doi: 10.1269/jrr.0616. [DOI] [PubMed] [Google Scholar]
- 17.Sholom S, Desrosiers M, Chumak V, et al. UV effects in tooth enamel and their possible application in EPR dosimetry with front teeth. Health Phys. 2010;98:360–8. doi: 10.1097/01.HP.0000348002.69740.bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toyoda S, Imata H, Romanyukha A, et al. Toward high sensitivity ESR dosimetry of mammal teeth: the effect of chemical treatment. J Radiat Res. 2006;47:A71–4. doi: 10.1269/jrr.47.a71. [DOI] [PubMed] [Google Scholar]
- 19.Haskell EH, Hayes RB, Kenner GH. Preparation-induced errors in EPR dosimetry of enamel: pre- and post-crushing sensitivity. Appl Radiat Isot. 1996;47:1305–10. doi: 10.1016/s0969-8043(96)00163-7. [DOI] [PubMed] [Google Scholar]
- 20.ISO/IEC. Uncertainty of measurement—Part 3: guide to the expression of uncertainty in measurement (GUM: 1995) ISO/IEC GUIDE 98-3:2008: 23–4.