Figure 5. Circuit performance within the context of a living cell. (a).
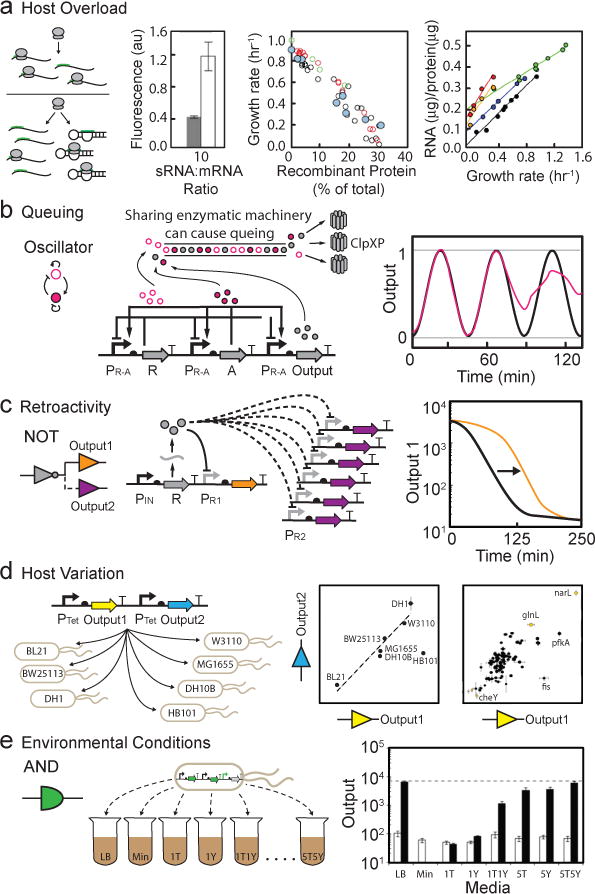
Recombinant protein expression can cause a growth defect by reducing the availability of host resources (e.g., RNAP and ribosomes). Here, synthetic sRNAs compete with mRNA for ribosomes to illustrate the impact of exogenous protein expression on host resource allocation. When sRNAs are produced (left graph, grey bars), ribosomes are titrated away from fluorescent protein mRNA and observed fluorescence is reduced relative to no sRNA (left graph, white bars)151. Center graph, colored circles represent the overexpression of different proteins in E. coli (blue: Pu promoter β-Galactosidase, red: T7 promoter β-Galactosidase, black: tac promoter ΔEF-Tu, green: bla promoter β-Lactamase)229. Right graph, colored circles represent growth of different bacterial strains as a function of rRNA supply (blue: E. coli 30°C, green: A. aerogenes 37°C, red: C. utilis 25°C, orange: C. utilis 30°C, black: N. crassa 30°C)229. (b) Queuing as a result of overloading the ClpXP protease machinery with proteins from a synthetic oscillator. The graph shows the difference between expected (black) and measured (red) dynamics for an oscillator affected by queuing166. (c) An additional output (PR2) on a high copy plasmid is added to the NOT gate. This causes retroactivity, which alters the activation dynamics of the original output (PR1) (black line: original dynamic response, orange line: retroactive effect)195. (d) One plasmid with two reporter proteins is transformed into different E. coli strains. The ratio of expression varies in some strains (left graph: wild type E. coli strains, right graph: KEIO collection knockouts)196. (e) Different media impact the performance of an AND gate based on T7 RNAP2,102. Data are shown for the circuit in the absence (white) and presence (black) of both inputs in different medias (LB: luria broth, Min: minimal media, #T and/or #L: minimal media supplemented with tryptone (#T = #g/L) or yeast extract (#Y = #g/L).
