Figure 7.
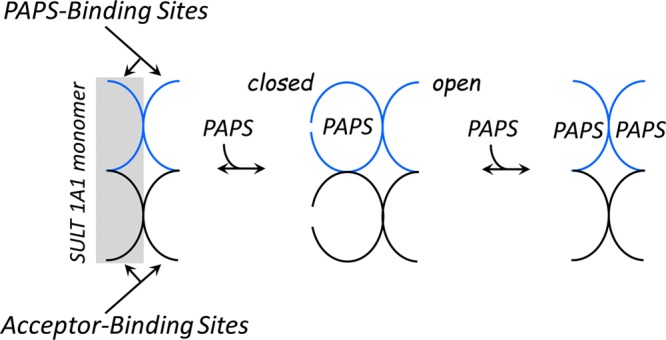
Coupling of PAPS binding and cap closure in SULT1A1. The ligand binding sites of the unliganded enzyme are open and can receive ligands. Binding of the first PAPS molecule closes both the PAPS and acceptor binding sites of the subunit to which PAPS has bound. In this configuration, PAPS cannot escape and only small acceptors can enter unless the enzyme isomerizes to the open form (not shown), which is unfavorable (Kiso = 26 in favor of the closed state). Consequently, the singly PAPS-bound configuration favors small acceptors. As the second PAPS molecule binds, all of the binding sites open, thus alleviating the catalytic bias against large substrates, and each subunit turns over 4-fold faster.
