Figure 1.
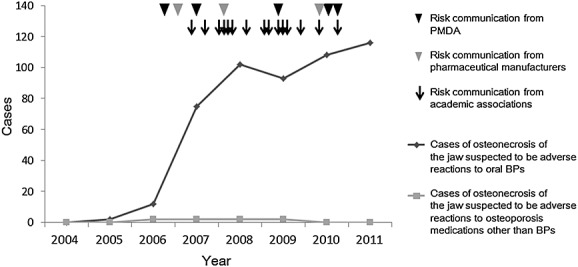
Trends in the number of ONJ cases per year reported to the Drug Adverse Reactions Reporting System of the PMDA and risk communication activities. Legend: The cases of ONJ that were suspected adverse reactions to oral bisphosphonates and those that were suspected adverse reactions to other agents for osteoporosis, reported to the Drug Adverse Reactions Reporting System of the PMDA, are shown as a dark gray line and a light gray line, respectively. Black arrowhead: risk communication from the PMDA; gray arrowhead: risk communication from pharmaceutical manufacturers; arrow: risk communication from academic associations
