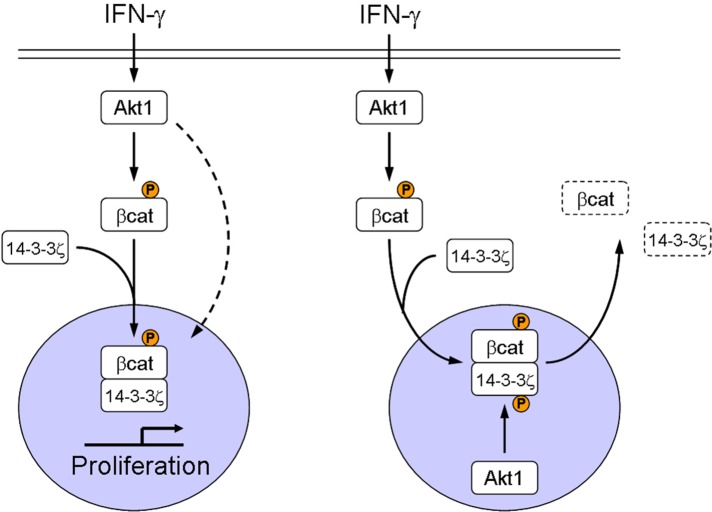
Nuclear Akt1 phosphorylates 14.3.3ζ at serine 58 to inhibit β-catenin transactivation. The results outline a dual function of Akt1, which suppresses intestinal epithelial cell proliferation during intestinal inflammation.
Abstract
The proinflammatory cytokine interferon γ (IFNγ ) influences intestinal epithelial cell (IEC) homeostasis in a biphasic manner by acutely stimulating proliferation that is followed by sustained inhibition of proliferation despite continued mucosal injury. β-Catenin activation has been classically associated with increased IEC proliferation. However, we observed that IFNγ inhibits IEC proliferation despite sustained activation of Akt/β-catenin signaling. Here we show that inhibition of Akt/β-catenin–mediated cell proliferation by IFNγ is associated with the formation of a protein complex containing phosphorylated β-catenin 552 (pβ-cat552) and 14.3.3ζ. Akt1 served as a bimodal switch that promotes or inhibits β-catenin transactivation in response to IFNγ stimulation. IFNγ initially promotes β-catenin transactivation through Akt-dependent C-terminal phosphorylation of β-catenin to promote its association with 14.3.3ζ. Augmented β-catenin transactivation leads to increased Akt1 protein levels, and active Akt1 accumulates in the nucleus, where it phosphorylates 14.3.3ζ to translocate 14.3.3ζ/β-catenin from the nucleus, thereby inhibiting β-catenin transactivation and IEC proliferation. These results outline a dual function of Akt1 that suppresses IEC proliferation during intestinal inflammation.
INTRODUCTION
The intestinal epithelium functions as a barrier that separates luminal contents from underlying tissue compartments. It is now evident that an intact epithelium is essential for maintaining the mucosal barrier function. Thus a balance between cell proliferation, migration, and apoptosis maintains epithelial homeostasis and directly contributes to regulation of barrier function. It is well appreciated that epithelial homeostasis is perturbed in a number of inflammatory disorders in which elevated mucosal proinflammatory cytokines have been shown to compromise the epithelial barrier. We reported that, in addition to disruption of barrier function, the proinflammatory cytokines interferon γ (IFNγ) and tumor necrosis factor (TNFα) exert biphasic effects on epithelial proliferation by transactivating β-catenin signaling pathways (Nava et al., 2010).
Although numerous signaling pathways have been shown to play roles in the regulation of epithelial homeostasis, the mechanisms behind the proinflammatory cytokine–mediated effects on epithelial equilibrium are not understood. IFNγ is critical for the induction of cell-mediated immunity and is one of the main cytokines identified in the inflamed mucosa of patients with inflammatory bowel disease (IBD). Its presence has been associated with changes in intestinal epithelial cell (IEC) homeostasis under certain conditions, but the mechanisms underlying this process are not well characterized. IFNγ activates phosphoinositide 3-kinase (PI3K) in a variety of cell lines (Kaur et al., 2008), and PI3K regulates proliferation, cell growth, and differentiation of the intestinal epithelial cells (Laprise et al., 2002; Xie et al., 2005; Xie and Bikle, 2007; Mitra et al., 2012). PI3K, through its downstream target protein Akt, enhances β-catenin–mediated cell transactivation via direct phosphorylation of β-catenin at the Ser-552 residue (pβ-cat552; Fang et al., 2007; He et al., 2007) or by inhibiting its proteosomal degradation (Tian et al., 2005). Increased transactivation of pβ-cat552 downstream of Akt also depends on its association with members of the 14.3.3 family of proteins (Tian et al., 2004). Members of the 14.3.3 family are adaptor proteins that regulate several signaling pathways due to their ability to bind signaling molecules, including kinases, phosphatases, and transmembrane receptors (Gardino and Yaffe, 2011; Obsil and Obsilova, 2011; Smith et al., 2011). Thus 14.3.3 proteins represent good candidate molecules that could play an important role in regulating intestinal epithelial homeostasis downstream of IFNγ by controlling Akt/β-catenin signaling events.
Here we report that IFNγ can enhance or reduce intestinal epithelial cell proliferation by regulating Akt/β-catenin signaling. We demonstrate that acute exposure of IEC to IFNγ resulted in Akt-mediated β-catenin phosphorylation, which facilitates its association with 14.3.3ζ. We show that association of β-catenin with 14.3.3ζ increases its stability and induces β-catenin transactivation, which, in turn, promotes Akt1 expression. We demonstrate that increased Akt1 protein levels result in nuclear accumulation of active Akt (pAkt308), which phosphorylates 14.3.3ζ, resulting in relocalization of β-catenin/14.3.3ζ protein complexes from the nucleus to the cytosol and inhibition of cell proliferation. These findings have implications for understanding the direct consequence of inhibited epithelial cell proliferation by proinflammatory cytokines in the mucosa of IBD patients.
RESULTS
IFNγ inhibits β-catenin transactivation in IECs
During inflammation, homeostatic properties of the epithelium are compromised, and it is well appreciated that deleterious effects of inflammation on epithelial homeostasis are mediated in part by TNFα and IFNγ (Koch and Nusrat, 2012). Nuclear translocation of β-catenin and T-cell factor/lymphoid enhancer factor transactivation controls IEC homeostasis, which is altered during inflammation by proinflammatory cytokines (Nava et al., 2010). Although one would predict increased epithelial proliferation (Seno et al., 2009) as part of the reparative response to inflammation-induced epithelial damage, our previous results suggested that sustained cytokine exposure was associated with suppression of epithelial proliferation.
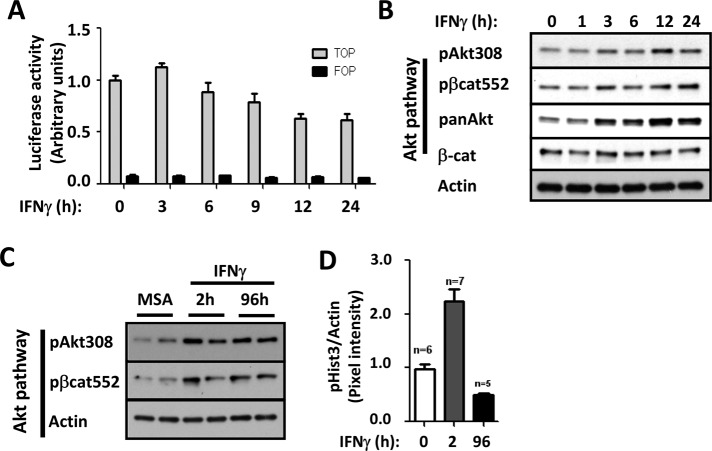
To understand the mechanisms by which of β-catenin activity and epithelial proliferation are influenced by IFNγ, we used complementary in vitro and in vivo approaches. Using a cultured intestinal epithelial cell line SW480, we evaluated the influence of IFNγ treatment on β-catenin transactivation by measuring TOPflash reporter activity (Korinek et al., 1997). IFNγ exposure resulted in an initial increase in β-catenin transactivation (3 h), followed by a steady decline over the next 24 h of cytokine treatment, suggesting transient β-catenin transactivation by the cytokine (Figure 1A). In agreement with our previous results using a model intestinal epithelial cell line, T84 (Nava et al., 2010), exposure of epithelial cells to IFNγ resulted in a biphasic proliferative response, with an initial increase in 5-ethynyl-2′-deoxyuridine incorporation (6–12 h), followed by reduced proliferation at 24 h (Supplemental Figure S1A). Although decreased activation of classical Wnt/β-catenin signaling was observed after IFNγ treatment (as we previously reported; Nava et al., 2010), increased activation of the Akt/β-catenin signaling was observed, as shown by the presence of high levels of pAkt308 and its downstream target protein, pβ-cat552 (Figure 1B and Supplemental Figure S1B). Moreover, Western blot analysis of colonic mucosal lysates of C57BL/6 mice that had received intraperitoneal injections of IFNγ also revealed increased activation of Akt/β-catenin signaling within 2 h, an effect that was also observed when mice were continuously exposed to IFNγ for 96 h (Figure 1C and Supplemental Figure S2). However, as shown in Figure 1D, increased IEC cell proliferation was only observed 2 h after IFNγ treatment. In fact, continuous exposure of IECs to IFNγ for 96 h resulted in a clear reduction in cell proliferation (Figure 1D). These findings suggested that sustained activation of Akt/β-catenin downstream of IFNγ signaling exerts biological effects that extend beyond increasing epithelial cell proliferation during inflammation.
FIGURE 1:
IFNγ induces transient transactivation of β-catenin in IECs. Effects of IFNγ in β-catenin transactivation (A) and Akt/β-catenin signaling pathway in vitro (B) and in vivo (C) were evaluated by TOPflash assays and Western blot analysis in SW480 cells and colonic mucosa of C57BL/6J mice, respectively. IFNγ was added 3–24 h before SW480 cells were processed. C57BL/6J mice were injected intraperitoneally with IFNγ for 2 h or vehicle alone (mouse serum albumin [MSA]). Transfections were performed in triplicate, and the means ± SD are shown (n = 3). Specific antibodies against pAkt308 and pβ-cat552 for Akt signaling pathway activation were used. Pan-Akt antibody was used to detect Akt total levels. Actin was used as a loading control. (D) The effect of IFNγ in IEC proliferation was evaluated by analyzing pHist3 expression in the mucosa of mice exposed to the cytokine for 2 and 96 h. Bar graph obtained of the densitometric analysis. pHist3 levels were normalized to actin.
IFNγ-mediated Akt activation promotes 14.3.3ζ and pβ-cat552 association and β-catenin redistribution
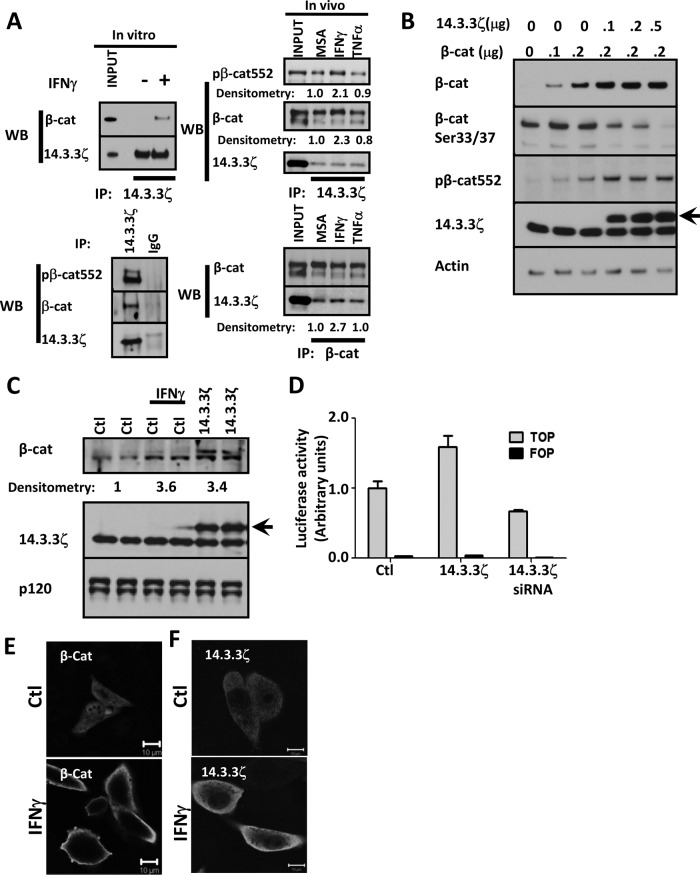
To understand the influence of sustained Akt/β-catenin activation on epithelial cell proliferation, we investigated the mechanism by which Akt controls β-catenin transactivation downstream of IFNγ. IECs express two isoforms of Akt, Akt1 and Akt2 (Brazil et al., 2004). Active Akt1 has been shown to directly phosphorylate β-catenin at serine 552 (pβ-cat552) to promote β-catenin association with an adaptor protein 14.3.3ζ, which, in turn, stabilizes and activates β-catenin (Tian et al., 2004). Because IFNγ influences IEC proliferation, we examined whether IFNγ exposure modulates association of β-catenin with 14.3.3ζ. Indeed, as shown in Figure 2A, injection of mice with IFNγ or addition of IFNγ to cultured IECs for 1 h resulted in an increase in coimmunoprecipitation of β-catenin with 14.3.3ζ in IECs. Furthermore, β-catenin that was complexed with 14.3.3ζ was phosphorylated at serine 552 (Figure 2A). In contrast, there was no effect on the association of both molecules after TNFα treatment in vivo (Figure 2A). Next we analyzed the effects of 14.3.3ζ on the stability of β-catenin, using an in vitro model. For these studies, we used CHO-K1 cells because they lack E-cadherin and express low levels of β-catenin due to its proteosomal degradation (Supplemental Figure S3). As shown in Figure 2B, expression of exogenous β-catenin in CHO-K1 cells along with increasing concentrations of 14.3.3ζ resulted in a corresponding increase in β-catenin and pβ-cat552. Under these conditions, we also observed a decrease in the phosphorylation β-catenin at serine 33 (pβ-cat33; Figure 2B), which has been shown to generate a recognition site for β-Trcp, resulting in the ubiquitination and degradation of soluble β-catenin (Liu et al., 1999). We then evaluated the role of Akt1 and 14.3.3ζ in regulating the stability of β-catenin. Consistent with the role of Akt1 and 14.3.3ζ in regulating β-catenin stability, small interfering RNA (siRNA)–mediated down-regulation of endogenous 14.3.3ζ or Akt1 resulted in reduced β-catenin protein levels (Supplemental Figure S4A). Furthermore, pharmacologic inhibition of Akt (Akt inhibitor VIII) also resulted in decreased β-catenin protein in control cells (Supplemental Figure S4B) and cells exposed to IFNγ for 12 h (unpublished data). Inhibition of Akt was verified by Western blot for pAkt308 (Supplemental Figure S4B). Moreover, as shown in Figure 2C, cytokine treatment or up-regulation of 14.3.3ζ resulted in increased endogenous β-catenin protein levels (3.6- and 3.4-fold increase, respectively). Consistent with previous reports (Tian et al., 2004), we observed that increased expression of 14.3.3ζ promoted β-catenin transactivation. Conversely, down-regulation of 14.3.3ζ inhibited β-catenin transactivation (Figure 2D). The influence of IFNγ treatment on localization of 14.3.3ζ and β-catenin was evaluated in IECs by immunofluorescence labeling. In control cells, immunofluorescence labeling demonstrated that β-catenin is evenly distributed in both nuclear and cytosolic compartments but is mainly present in the cytoplasm after treatment with IFNγ for 12 h (Figure 2E). Similarly, IFNγ treatment for 12 h induced accumulation of 14.3.3ζ in the cytoplasm (Figure 2F). Taken together, these findings suggested that 14.3.3ζ may play an important role in regulating the cellular distribution of β-catenin and its function after stimulation with IFNγ.
FIGURE 2:
IFNγ promotes the association of β-catenin with 14.3.3ζ. (A) Association of β-catenin with 14.3.3ζ was analyzed by coimmunoprecipitation assays. 14.3.3ζ and control immunoglobulin G (IgG) were immunoprecipitated from fresh lysates obtained from SW480 cells, control or treated with IFNγ for 1 h. 14.3.3ζ was immunoprecipitated from IECs isolated from murine intestinal mucosa exposed for 2 h to vehicle (MSA), IFNγ, and TNFα. Immunoprecipitates were blotted for β-catenin, pβ-cat552, and 14.3.3ζ. Densitometric analysis of β-catenin, pβ-cat552, and 14.3.3ζ. (B) The effect of 14.3.3ζ on β-catenin stabilization was analyzed in CHO cells. Confluent monolayers of CHO cells were transfected with 0.1–0.2 μg/ml β-catenin–expressing vector in presence of increasing concentrations of a 14.3.3ζ-expressing vector (arrow). Cell lysates were collected in RIPA lysis buffer and equal amounts of proteins loaded and analyzed by Western blotting. Actin was used as a loading control. (C) The effect of IFNγ and 14.3.3ζ (arrow) overexpression on endogenous β-catenin stability was determined by Western blot in CHO cells. Relative densitometric values were normalized with respect to the controls. p120 catenin was used as a loading control. (D) The effect of 14.3.3ζ expression on β-catenin transactivation was analyzed by TOPflash assays. SW480 cells were transfected with a vector expressing 14.3.3ζ or siRNA targeting 14.3.3ζ and luciferase expression determined. The cellular distribution of β-catenin (E) and 14.3.3ζ (F) was analyzed by immunofluorescence in SW480 cells that were exposed to vehicle (Ctl) or IFNγ for 12 h. Nuclei are blue. Bar, 10 μm.
Phosphorylation of 14.3.3ζ at serine 58 after IFNγ treatment promotes relocalization of β-catenin/14.3.3ζ
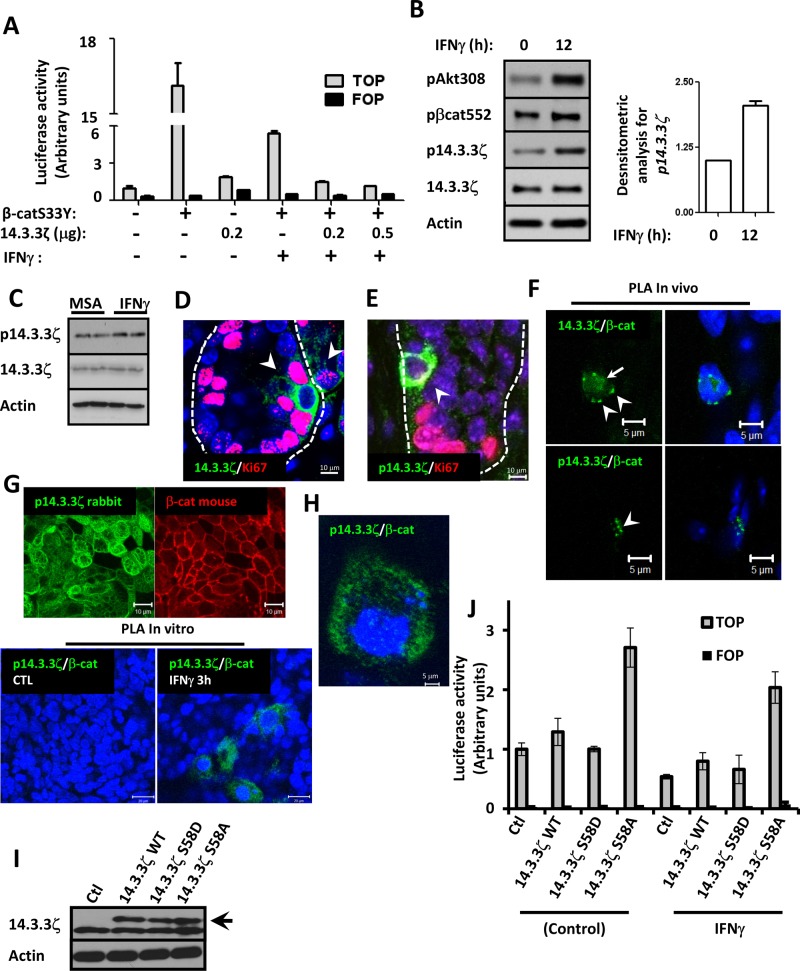
The mechanism of inhibition of β-catenin signaling by 14.3.3ζ after IFNγ exposure was further investigated. TOPflash reporter assays demonstrated that expression of stabilized β-catenin (β-catS33Y) in SW480 cells increased TOPflash activity by ∼10-fold, and 14.3.3ζ overexpression alone induced an approximately twofold increase in β-catenin transactivation (Figure 3A). Coexpression of 14.3.3ζ and β-catS33Y did not have additive effects (unpublished data). However, when cells expressing β-catS33Y were treated with IFNγ for 24 h, β-catenin transactivation was reduced by ∼60%. Expression of increasing amounts of 14.3.3ζ had an additive effect on IFNγ-mediated suppression of β-catenin transactivation (Figure 3A). Unexpectedly, β-catenin transactivation was not affected in cells exposed to IFNγ for short periods of time (3–6 h), even in cells overexpressing 14.3.3ζ (unpublished data). These results suggested that inactivation of β-catenin transactivation by14.3.3ζ downstream of IFNγ requires additional posttranslational modifications.
FIGURE 3:
Decreased IEC β-catenin transactivation in response to IFNγ is associated with phosphorylation of 14.3.3ζ at serine 58. (A) Regulation of β-catenin transactivation by 14.3.3ζ was analyzed in SW480 cells treated with IFNγ by TOPflash assay. Cells were transfected with 0.2 μg/ml vector expressing active β-catS33Y alone or in conjunction with 0.2 or 0.5 μg/ml 14.3.3ζ. IFNγ was added 12 h posttransfection and samples collected 24 h post cytokine treatment. Experiments were performed in triplicate in two different cell passages. Means ± SD of a representative experiment. (B) Phosphorylation status of 14.3.3ζ (Ser-58), β-catenin (Ser-552), Akt (Thr-308), and total protein levels of 14.3.3ζ was analyzed in SW480 cells exposed to IFNγ (12 h) by Western blot. Actin was used as a loading control. Densitometric analysis of p14.3.3ζ is shown in the graph (n = 3). (C) The expression of 14.3.3ζ and p14.3.3ζ in the intestinal colonic mucosa of mouse injected with IFNγ was analyzed by Western blot. Actin was used as a loading control. The distribution of 14.3.3ζ (D) and p14.3.3ζ (E) at the colonic crypts of C57BL/6N animals was analyzed by immunofluorescence. Bar, 10 μm. Nuclei are blue. Proliferating cells are marked with Ki67 (red). Crypt plane is marked by a discontinuous line. (F) PLA assays for 14.3.3ζ/β-catenin (green) and p14.3.3ζ/β-catenin (green) were performed in colonic mucosa of C57BL/6N animals. Scale bar, 5 μm. Nuclei are blue. (G) Immunofluorescence labeling for p14.3.3ζ (green) and β-catenin (red) and PLA assay for p14.3.3ζ/β-catenin (green) were performed in T84 cells exposed to IFNγ for 3 h. Scale bar, 10 μm. Nuclei are blue. (H) PLA assay for p14.3.3ζ/β-catenin (green) performed in T84 cells. High magnification of T84 cells exposed to IFNγ for 3 h. Scale bar, 2 μm. Nuclei are blue. (I) Overexpression of 14.3.3ζ mutants does not affect endogenous 14.3.3ζ protein levels. SW480 cells were transfected with 200 ng of plasmid expressing empty vector, 14.3.3ζ WT, 14.3.3ζ S58D, and 14.3.3ζ S58A overnight and 14.3.3ζ expression analyzed by Western blotting of whole-cell lysates. Black arrow marks the overexpressed proteins. (J) 14.3.3ζ S58A prevents inhibition of β-catenin transactivation in IECs exposed to IFNγ. The effect of 14.3.3ζ WT, 14.3.3ζ S58D, and 14.3.3ζ S58A on β-catenin transactivation mediated by IFNγ was evaluated by TOPflash luciferase assays in SKCO15 cells. IFNγ was added 12 h before cells were processed for the TOPflash luciferase assay. Values were normalized to empty vector. Transfections were performed in triplicate, and the means ± SD are shown (n = 3).
Previous studies showed that phosphorylation of the N-terminal region of 14.3.3ζ at serine 58 (p14.3.3ζ) by serine/threonine kinases results in inhibition of function (Megidish et al., 1998). We thus analyzed phosphorylation of 14.3.3ζ in cells treated with IFNγ at later time points. As shown in Figure 3B, increased levels of p14.3.3ζ were observed in IECs exposed to IFNγ for 12 h, and phosphorylation levels corresponded with inhibition of β-catenin transactivation after cytokine treatment (Figure 1A). We then performed in vivo experiments to investigate the expression and localization of 14.3.3ζ and p14.3.3ζ in the mucosa of C57BL/6J mice after intraperitoneal injection with IFNγ. As shown in the Western blots in Figure 3C, increased p14.3.3ζ was seen in the intestinal mucosa from mice 3 h after IFNγ administration, whereas total levels of 14.3.3ζ protein remained unchanged. Immunofluorescence labeling of colonic mucosa identified 14.3.3ζ and p14.3.3ζ protein in crypt and surface epithelial cells (Supplemental Figure S5). However, increased 14.3.3ζ and p14.3.3ζ protein was identified in nonproliferating crypt epithelial cells that exhibited negative staining for Ki67 (Figure 3, D and E). Of interest, IFNγ administration increased the number of crypt epithelial cells that exhibited strong labeling for 14.3.3ζ and p14.3.3ζ but lack Ki67 staining (Supplemental Figure S5). To investigate the relationship of β-catenin with 14.3.3ζ in crypt epithelial cells, we analyzed association of these proteins by proximity ligation assay (PLA), a method that analyzes protein–protein interactions with high specificity and sensitivity. Secondary antibodies are coupled to complementary oligonucleotides, and if proteins are in close proximity, the complimentary DNA strands hybridize and the signal is amplified using fluorescently labeled oligonucleotides, leading to distinct fluorescent spots in the sites of interaction (Soderberg et al., 2006; Jarvius et al., 2007). As shown in Supplemental Figure S6, β-catenin is distributed predominantly in the basal membrane of colonic epithelial cells, whereas 14.3.3ζ and p14.3.3ζ localize in the cytoplasm. PLA assay revealed that 14.3.3ζ and β-catenin are in close proximity in the cytoplasm (arrowhead) as well as in the nucleus (arrow) (Figure 3F). In contrast, p14.3.3ζ and β-catenin protein complexes were only observed in the cytoplasm (Figure 3F, arrowhead). To further verify the in vivo observations, we analyzed association of β-catenin with p14.3.3ζ by PLA using a model intestinal epithelial cell line, T84. As shown in Figure 3G, p14.3.3ζ and β-catenin are distributed in the lateral plasma membrane and cytoplasm of IECs. However, PLA assay demonstrated association of p14.3.3ζ with β-catenin only in the cytoplasm of IECs treated with IFNγ (Figure 3, G and H). Next the contribution of 14.3.3ζ phosphorylation at serine 58 in the regulation of β-catenin signaling downstream of IFNγ was investigated using TOPflash reporter assays. The influence of expressing a phosphomimetic point mutant of 14.3.3ζ (S58D) and a phosphorylation-defective mutant of 14.3.3ζ (S58A) on β-catenin transactivation was evaluated by analysis of TOPflash luciferase activity in IECs. Thus equal amounts of 14.3.3ζ wild type (WT), 14.3.3ζ S58D, and 14.3.3ζ S58A were transfected in IECs (Figure 3I). The expression of 14.3.3ζ mutants did not affect 14.3.3ζ protein levels of endogenous protein (Figure 3I). As shown in Figure 3J, cells transfected with 14.3.3ζ WT showed a modest increase in TOPflash luciferase activity (1.00 ± 0.105 vs. 1.29 ± 0.23), whereas we did not observe an influence on β-catenin transactivation in cells overexpressing 14.3.3ζ S58D (1.00 ± 0.105 and 1.01 ± 0.045). However, the expression of 14.3.3ζ S58A enhanced β-catenin transactivation (1.00 ± 0.105 vs. 2.70 ± 0.33). IFNγ treatment for 12 h decreased β-catenin transactivation in control cells (46%, 0.54 ± 0.038) and in cells expressing 14.3.3ζ WT (39%, 0.79 ± 0.14), 14.3.3ζ S58D (34%, 0.66 ± 0.23). and 14.3.3ζ S58A (24%, 2.03 ± 0.26). In the presence of 14.3.3ζ S58A, the influence of IFNγ on β catenin transactivation was less prominent than with the other conditions. Taken together, these results suggest that phosphorylation of 14.3.3ζ mediates relocalization of β-catenin from the nucleus to the cytoplasm to inhibit β-catenin transactivation and IEC proliferation downstream of IFNγ.
Akt1 induces phosphorylation of 14.3.3ζ at serine 58
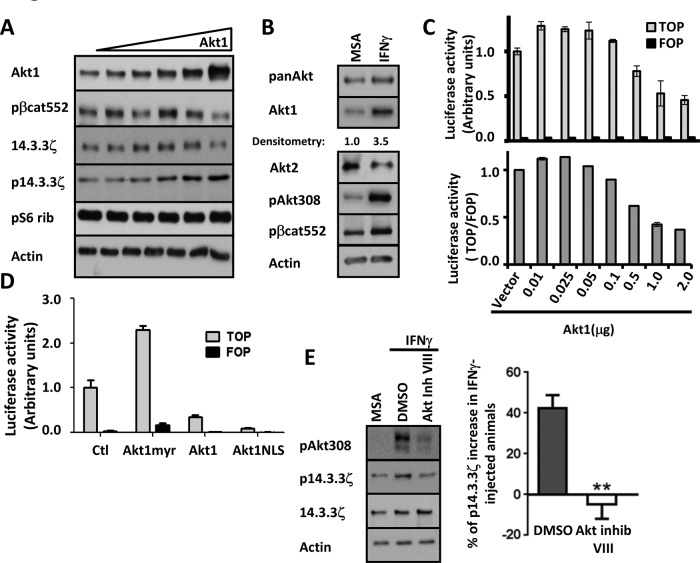
Experiments were performed to identify the kinase that mediates phosphorylation of 14.3.3ζ. Because Akt1 has been shown to phosphorylate 14.3.3ζ at serine 58 (Powell et al., 2002), we analyzed the role of Akt1 in generation of the p14.3.3ζ. Increasing concentrations of Akt1 were transfected in SW480 cells, and the expression of p14.3.3ζ was quantified. As shown in Figure 4A, whereas low concentrations of Akt1 did not influence p14.3.3ζ, expression of high amounts of the kinase increased levels of p14.3.3ζ. Of interest, we also observed that total levels of 14.3.3ζ and pβ-cat552 were increased when small amounts of Akt1 were expressed, and high levels of Akt1 diminished 14.3.3ζ and pβ-cat552 protein levels (Figure 4A), suggesting a complex relationship between Akt1 protein levels and phosphorylation/stabilization of 14.3.3ζ and pβ-cat552. Of importance, no changes were observed in the phosphorylation of an unrelated protein pS6 rib after overexpression of Akt1 (Figure 4A). To corroborate these findings, we analyzed the levels of Akt protein in the mucosa of IFNγ-treated mice. As shown in Figure 4B, IFNγ treatment resulted in increased Akt1 protein levels in lysates from the intestinal mucosa of mice exposed to IFNγ for 1–3 h. In contrast, the same IFNγ treatment resulted in decreased Akt2 protein levels. Immunofluorescence staining analyses of intestinal mucosa revealed that Akt1 was enriched in the cytosol of epithelial cells in the base of nonproliferative crypt IEC in control and IFNγ-treated conditions, suggesting that increased Akt1 may contribute to inhibition of intestinal epithelial cell proliferation (Supplemental Figure S7).
FIGURE 4:
Phosphorylation of 14.3.3ζ at serine 58 requires up-regulation of Akt1 protein levels. (A) The effect of increasing concentrations of Akt1 on phosphorylation of 14.3.3ζ was analyzed in cell lysates of SW480 cells. Akt1, 14.3.3ζ, p14.3.3ζ, and pS6 Rib were analyzed by Western blot. Actin was used as a loading control. (B) The expression of Akt1 and Akt2 after IFNγ treatment was analyzed by Western blotting lysates of colonic tissue of mice injected with IFNγ. Western blots were performed using antibodies against pan-Akt, Akt1, Akt2, pAkt308, and pβ-cat552. Actin was used as loading control. (C) The role of Akt1 expression in β-catenin transactivation was evaluated in SW480 cells transfected with 0.01–2 μg/ml vector expressing Akt1. β-Catenin transactivation was evaluated by TOPflash assay. TOP vs. FOP and TOP/FOP activity are plotted. (D) Effects of Akt1 subcellular distribution on β-catenin–mediated transcriptional activity was evaluated by TOPflash assay in SW480 cells. Cells were transfected with 0.75 μg/ml Akt1-myr, Akt1, or Akt-NLS constructs in serum-free medium. (E) The expression of p14.3.3ζ was analyzed in colonic cell lysates of IFNγ-injected animals that were pretreated with dimethyl sulfoxide or Akt inhibitor VIII. Densitometric values were normalized to actin and are presented as a graph (**p < 0.0001).
To further gain insight into the contribution of Akt1 protein levels in inhibiting β-catenin signaling, we examined the effect of increasing concentrations of Akt1 on β-catenin transactivation. Expression of different amounts of Akt (Akt-HA) in SW480 cells had differential effects on β-catenin–mediated TCF reporter activity. As shown in Figure 4C, expression of low levels of Akt1 increased β-catenin transactivation, whereas further increased Akt1 expression repressed β-catenin transactivation, analogous to that observed with IFNγ (Figure 1A). We hypothesized that Akt1 protein levels may contribute to the generation of subcellular pools of Akt1 that differentially control β-catenin transactivation. Under this scenario, low cellular levels of Akt1 would result in preferential localization of pAkt308 in the cytoplasm and plasma membrane, whereas increased Akt levels are associated with distribution of Akt1 protein in the nucleus. To test this possibility, we used a gain-of-function approach to overexpress Akt1 mutant proteins that are preferentially targeted to one of these subcellular compartments (Supplemental Table S1) and determined their effect(s) on β-catenin transactivation. As previously reported and shown in Figure 4D, increased β-catenin transactivation was observed in SW480 cells expressing membrane-associated Akt1 (Akt1–myristoylation signal [myr]; Li et al., 2008). Conversely, overexpression of unmodified Akt1 resulted in suppressed TOPflash reporter activity. Furthermore, expression of a mutant Akt1 with two nuclear localization signals (NLSs) in tandem (Wang and Brattain, 2006) was even more efficient in suppressing β-catenin transactivation, as previously reported (Figure 4D; Li et al., 2008), and is consistent with nuclear Akt1-mediated inhibition of β-catenin transactivation. Because the foregoing results support a specific role of Akt1 in the regulation of β-catenin transactivation in IEC, we investigated whether the redistribution of 14.3.3ζ/pβ-cat552 from nucleus to cytosol (Figure 2G) was mediated by Akt1. Akt1, pAkt308, 14.3.3ζ, and pβ-catenin were analyzed in nuclear fractions of SW480 transiently overexpressing Akt1. Akt1 overexpression increased pAkt308 and pβ-cat552 protein levels in total cell extracts of SW480 without influencing 14.3.3ζ and β-catenin protein levels (Supplemental Figure S8A). Overexpression of Akt1-HA in SW480 cells increased pAkt308 in the nucleus, accompanied by decreased levels of nuclear 14.3.3ζ, without influencing pβ-cat552 (Supplemental Figure S8B). Given that SW480 cells have APC mutations that can influence pβ-cat552, we further characterized the influence of Akt1 overexpression in CHO cells expressing E-cadherin (CHO-Ecad). CHO-Ecad cells were used because they have high levels of endogenous β-catenin. As shown in Supplemental Figure S8B, increased Akt1 expression promoted accumulation of nuclear pAkt308, which was associated with reduced nuclear 14.3.3ζ and pβ-cat552 in CHO-Ecad cells. These results suggest that increased nuclear Akt1 decreases 14.3.3ζ and pβ-cat552 in the nucleus. We next determined whether IFNγ increased nuclear pAkt308, which modulates pβ-cat552 and 14.3.3ζ localization. Similar to our previous findings, IFNγ treatment for 12–24 h resulted in reduced nuclear 14.3.3ζ, which correlated with the increased pAkt308 in nuclear fractions of SW480 and CHO-Ecad cells (Supplemental Figure S8C). However, decreased pβ-cat552 was observed only in CHO-Ecad cells (Supplemental Figure S8C).
Next we investigated the role of Akt in controlling 14.3.3ζ nuclear localization in response to IFNγ. As shown in Supplemental Figure S9, IFNγ treatment (1–3 h) increased levels of nuclear 14.3.3ζ in cells that were treated with Akt inhibitor VIII, but we did not observe a change in 14.3.3ζ protein in IFNγ-treated monolayers. These results suggested that Akt activity influences nuclear levels of 14.3.3ζ. To demonstrate the role of Akt in phosphorylation of 14.3.3ζ, we performed in vivo analyses of p14.3.3ζ protein in intestinal mucosa of mice after IFNγ injection in the presence and absence of Akt inhibitor VIII. As shown in Figure 4E, Akt inhibition prevented the increase in phosphorylation of 14.3.3ζ in mice after IFNγ administration. Taken together, the foregoing results suggested that phosphorylation of 14.3.3ζ induced by IFNγ treatment requires activation of Akt.
Akt1 is a target protein downstream of β-catenin in intestinal epithelial cells
Experiments were performed to investigate mechanism(s) by which IFNγ induces expression of Akt1. In colonic epithelial cells, Akt1 expression was reported to be induced by β-catenin transactivation (Dihlmann et al., 2005). Given our findings, we hypothesized that transactivation of β-catenin by Akt after IFNγ exposure at early time points (Figure 1A) was responsible for subsequent increases in Akt1protein expression. As shown in Figure 5A, IFNγ treatment for 2 h increased Akt1 mRNA expression in SW480 cells, and siRNA-mediated down-regulation of β-catenin inhibited this effect. We also investigated the possibility that β-catenin transactivation by Akt and 14.3.3ζ may regulate Akt1 expression after IFNγ treatment. Indeed, as shown in Figure 5B, IFNγ treatment resulted in an increase in Akt1 mRNA that was prevented by pharmacologic inhibition of Akt kinase activity. Furthermore, forced expression of 14.3.3ζ increased Akt1 mRNA levels that were further increased when the cells were treated with IFNγ (Figure 5B). Of importance, pharmacologic inhibition of Akt activity abrogated the increase in Akt1 mRNA mediated by 14.3.3ζ overexpression in the absence or presence of IFNγ (Figure 5B). Finally, we investigated the effect of 14.3.3ζ on the regulation of Dkk-1, a Wnt inhibitor known to be induced by IFNγ during inflammation (Nava et al., 2010). As shown in Supplemental Figure S10, up-regulation of DKK-1 mRNA levels was observed in IECs transiently expressing 14.3.3ζ. Thus, taken together, these results suggested that activation of β-catenin by Akt is necessary to induce expression of Akt1 and Dkk-1 to establish a feedback loop that inhibits Wnt/β-catenin and IEC proliferation.
FIGURE 5:
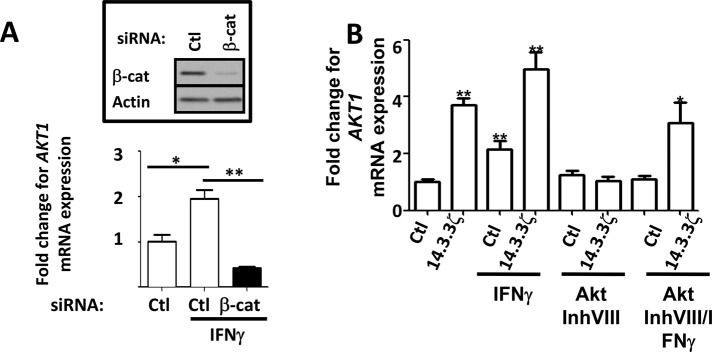
β-Catenin transactivation mediates Akt1 expression in IECs. (A) The effect of β-catenin down-regulation on Akt1 mRNA expression in response to IFNγ was analyzed by qRT-PCR. Down-regulation of β-catenin was achieved using a specific siRNA and analyzed by Western blot (inset). Experiments were performed in duplicate, and the means ± SD are shown. Akt1 mRNA levels were normalized to glyceraldehyde-3-phosphate dehydrogenase expression. *p < 0.005;**p < 0.001. (B) The effect of Akt/β-catenin signaling pathway on Akt1 mRNA expression was analyzed by qRT-PCR in control or IFNγ-treated SW480 cells. Activation of Akt/β-catenin was achieved by 14.3.3ζ overexpression or IFNγ treatment. A pharmacological inhibitor (Akt inhibitor VIII; 2.12 μM) was used to inhibit Akt. Experiments were performed in duplicate, and means ± SD are shown. *p < 0.005;**p < 0.001.
DISCUSSION
β-Catenin signaling plays a pivotal role in controlling the homeostatic property of intestinal epithelial crypts. As intestinal epithelial cells differentiate and migrate to the luminal surface of crypts, physiologic β-catenin activation and proliferation are inhibited. However, the mechanisms regulating this process are incompletely understood. It is well appreciated that proinflammatory cytokines such as IFNγ and TNFα modulate multiple aspects of epithelial homeostasis, in part through effects on β-catenin signaling (Nava et al., 2010; Capaldo et al., 2012). The relationship between intestinal epithelial homeostasis and cytokines is temporal in nature, depending on the extent and duration of the inflammatory response. Here we report a new mechanism that governs the stimulatory and inhibitory effects of IFNγ on β-catenin signaling to control intestinal epithelial proliferation. We provide evidence of the temporal interplay of Akt1 and a scaffold protein, 14.3.3ζ, in controlling β-catenin subcellular distribution, its transactivation, and finally, epithelial cell proliferation.
The role of Akt in regulation of β-catenin signaling is incompletely understood and controversial. From the findings in this study and others (Dihlmann et al., 2005; He et al., 2007; Li et al., 2008), it is likely that controversy over the role of Akt is related, in part, to type and duration of stimulus and cellular concentrations of Akt. Through analyses of the response of IECs to IFNγ treatment, we were able to demonstrate that Akt1 controls activation and inhibition of β-catenin signaling downstream of IFNγ and propose a hypothetical model in which Akt1 controls the fate of β-catenin through regulation of cellular localization of the complex 14.3.3ζ/pβ-cat552 (Figure 6). The model we propose predicts that PI3K activation by IFNγ (Kaur et al., 2008) results in the activation of Akt1. Active Akt phosphorylates β-catenin at serine 552. Such posttranslational events facilitate interaction of β-catenin with 14.3.3ζ, thereby promoting β-catenin stabilization and transactivation (Figure 6, left; Tian et al., 2004). As a consequence of this process, Akt1 protein levels increase. Elevated levels of Akt1 result in enrichment of pAkt308 within the nucleus, enhances phosphorylation of 14.3.3ζ at serine 58 by Akt (Powell et al., 2002), and its subsequent relocalization from nucleus to cytosol, where the molecules accumulate (Figure 3, D and E; Tian et al., 2004; He et al., 2007). It is therefore likely that the foregoing mechanism is responsible for the expulsion of 14.3.3ζ and pβ-cat552 from the nuclear compartment, resulting in inhibition of β-catenin transactivation and inhibition of IEC proliferation (Figure 6, right).
FIGURE 6:
Model showing IFNγ regulation of β-catenin.
The results in this study cannot exclude the possibility that increasing Akt1 protein levels by IFNγ could inhibit β-catenin signaling via other mechanisms. Indeed, up-regulation of Akt1 in response to IFNγ could promote association of 14.3.3ζ with Akt1, resulting in cytoplasmic sequestration of β-catenin, as shown in other models (Li et al., 2008). Moreover, the monomeric form of 14.3.3ζ generated after phosphorylation of 14.3.3ζ (Zhou et al., 2009; Sluchanko and Gusev, 2012) by Akt1 could enhance β-catenin proteasomal degradation, as reported for other proteins (Sato et al., 2011).
It is well established that synthesis of new proteins is a mechanism by which a negative feedback loop is generated to inhibit or terminate signaling and reestablish homeostasis (Jho et al., 2002; Niida et al., 2004). Thus activation of Wnt/β-catenin signaling can negatively regulate itself by promoting synthesis of inhibitory proteins such as Dkk1 and Axin2 (Jho et al., 2002; Lustig et al., 2002; Gonzalez-Sancho et al., 2005). Such newly synthesized proteins can then target other regulatory proteins. The present study suggests that a single molecule such as Akt1 can activate or inhibit β-catenin transactivation under specific conditions by controlling accessory molecules such as 14.3.3ζ. This mechanism is complementary to previously reported effects of Dkk1 and GBP1, which serve to inhibit β-catenin activation during inflammation (Nava et al., 2010; Capaldo et al., 2012). Of interest in the present study, we observed that 14.3.3ζ overexpression in IECs increased Dkk-1 mRNA, supporting a relationship of these β-catenin signaling inhibitory pathways (Supplemental Figure S10). Thus, in concert with other reports, these findings suggest that multiple mechanisms have evolved to prevent uncontrolled β-catenin signaling and epithelial proliferation (Ng et al., 2009; Nava et al., 2010). It is reasonable to assume that such mechanisms might play important roles in controlling molecular events that modulate proliferative responses under a variety of physiologic and pathological conditions. Although further studies are needed, it is clear from our results that understanding the mechanisms by which 14.3.3ζ controls cytosolic β-catenin activity could provide insight into mechanism(s) aimed at inhibiting epithelial cell proliferation in tumor cells in which β-catenin signaling is dysregulated.
Previous reports suggested synergy between the Akt and Wnt signaling pathways upstream of β-catenin (Fukumoto et al., 2001; Naito et al., 2005). However, our results demonstrate that during inflammation, transactivation of β-catenin by Akt and 14.3.3ζ results in reduced Wnt signaling (Nava et al., 2010; Figure 3). Given the findings in this study, we hypothesized that during inflammation, components of Wnt and Akt signaling cascades form part of a larger regulatory pathway that controls β-catenin transactivation. In fact, our results predict that increased expression of 14.3.3ζ by Wnt/β-catenin would trigger β-catenin transactivation via Akt, a mechanism that could be important in promoting cell differentiation rather than proliferation. This model is consistent with a previous report highlighting a role of β-catenin in controlling differentiation of human embryonic stem cells (Davidson et al., 2012).
In conclusion, the above results support the concept of spatiotemporal regulation of β-catenin as an essential mechanism in controlling intestinal epithelial cell homeostasis. We hypothesize that perturbation of upstream regulators of β-catenin such as Akt and 14.3.3ζ results in changes in the rate of intestinal epithelial proliferation in response to the proinflammatory cytokine IFNγ. Moreover, this study identifies 14.3.3ζ and Akt1 as important regulators of β-catenin signaling during inflammation.
MATERIALS AND METHODS
Antibodies and reagents
Recombinant human and mouse IFNγ were obtained from Peprotech (Rocky Hill, NJ) and used at 100 U/ml for in vitro treatment and 2.5 μg/kg of weight for in vivo experiments. Recombinant mouse TNFα was obtained from PeproTech and used at 0.5 μg/kg of weight for in vivo experiments. MISSION siRNA (Supplemental Table S2) was obtained from Sigma-Aldrich (St. Louis, MO) and transfected with Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Primers for quantitative real-time PCR (qRT-PCR; Supplemental Table S2) were purchased from Realtimeprimes.com (Elkins Park, PA). AKT inhibitor Akt inhibitor VIII was obtained from Calbiochem (Darmstadt, Germany) and used according to the supplier's recommendation. Information about primary antibodies and constructs can be found in Supplemental Tables S3 and S4. Secondary antibodies were purchased from Invitrogen and Jackson ImmunoResearch (Westgrove, PA).
Cell culture
Intestinal epithelial cell model SW480 and CHO-K1 cells were grown in DMEM with 10% fetal calf serum and antibiotics. Cells were maintained in a humidified incubator with 5% CO2. For functional studies, cells were seeded onto collagen-coated, permeable filters (Costar, Tewksbury, MA) or glass coverslips.
Animal experiments
All procedures with animals were reviewed and approved by the Emory University Institutional Animal Care and Use Committee and by the Centro de Investigación y de Estudios Avanzados, Instituto Politécnico Nacional, Internal Committee for Care and Use of Laboratory Animals and were performed according to National Institutes of Health and Consejo Nacional de Ciencia y Tecnología criteria. C57Bl/6J mice were obtained from the Jackson Laboratories (Bar Harbor, ME). Animals were housed in a standard day-and-night cycle, with free access to food and water. IFNγ was dissolved in a carrier made with 0.002% mouse serum albumin and injected intraperitoneally in male mice (20–25 g). Intestinal epithelial cells were isolated from washed colon segments by repeated incubation with 50 mM EDTA (Sigma-Aldrich) in Hanks’ balanced salt solution and protein extracted and analyzed by Western blot. Protein localization was visualized by indirect immunofluorescence staining.
Western blot assays
Samples were collected in RIPA lysis buffer (150 mM NaCl, 1% NP-40, 0.5% deoxycholic acid, 0.1% SDS, 50 mM Tris, pH 8.0) supplemented with protease and phosphatase inhibitors (Sigma-Aldrich), sonicated, and cleared by centrifugation. Protein concentration was determined with a bicinchoninic acid protein assay, and samples were boiled in SDS sample buffer with 50 mM dithiothreitol. Equal amounts of protein were separated by SDS–PAGE and transferred onto nitrocellulose membranes. Membranes were blocked for 1 h with 2% (wt/vol) dry milk or bovine serum albumin (BSA) in Tris-buffered saline containing 0.1% Tween-20 and incubated with primary antibodies in blocking buffer overnight at 4°C.
Immunofluorescence microscopy
Tissue sections were fixed with paraformaldehyde (15 min) and then permeabilized with 100% ethanol (20 min, −20°C). Samples were then blocked with 2% (wt/vol) BSA for 1 h and incubated with primary antibodies overnight at 4°C. After incubation with fluorophore-labeled secondary antibodies for 1 h, nuclei were stained with ToPro-3 iodide (Molecular Probes, Carlsbad, CA), and coverslips were mounted in p-phenylene. Images were taken on an LSM 510 confocal microscope (Zeiss, Jena, Germany) with Plan-Neofluar 1003/1.3 oil, 403/1.3 oil, and 203/0.5 dry objectives, with software supplied by the vendor.
Real-time PCR
Total RNA was isolated from purified IEC using the Qiagen RNeasy Mini Kit (Germantown, MD), according to the manufacturer's protocol, with on-column DNase digestion using the RNase-Free DNase set. cDNA was generated using the Superscript First-Strand Synthesis System for real-time (RT) PCR and random hexamer primers (Invitrogen), according to the manufacturer's protocol. cDNA was used as a template for qRT-PCR using SYBR Green Master Mix (Bio-Rad, Hercules, CA) and specific primers (Supplemental Table S2). PCR and analysis were performed using a MyiQ iCycler (Bio-Rad), and gene expression was calculated relative to Gapdh.
Cellular fractionation
Cytoplasmic and nuclear extracts were prepared using the NE-PER Nuclear and Cytoplasmic Extraction Kit (Thermo Scientific, Waltham, MA). The Thermo Scientific Subcellular Protein Fractionation Kit was used for cytoplasmic, membrane, and nuclear-soluble fraction extracts. Both kits were used according to the manufacturer's protocol.
In situ proximity ligation assay
The Duolink in situ proximity ligation assay (Uppsala, Sweden) was performed according to the manufacturer's instructions.
Statistics
Dunnett's posttest after one-way analysis of variance or two-tailed Student's t test was used to analyze the data (GraphPad Software, La Jolla, CA). p < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Mattie Feasel, Caroline Addis, Clifton Huang, José Ayala Dávila, and Gretel Uriarte for technical assistance and A. Farkas for critical reading of the manuscript. This work is supported by grants from the National Institutes of Health (1R01DK097256 to T.L.D.; DK72564, DK61379, and DK79392 to C.A.P.; DK53202, DK55679, and DK59888 to A.N.; and DK64399, a National Institutes of Health Digestive Diseases Research Development Center tissue culture and morphology grant), the American Gastroenterological Association (Research Scholar Award to P.N.), the Crohn's and Colitis Foundation of America (Senior Research Award to T.L.D.; Career Development Award to P.N.; and Fellowship Award to S.K. and O.M.C.), a grant from the Consejo Nacional de Ciencia y Tecnología (175854 to P.N.D.), and the Glaxo-SmithKline International Award from the Japan Society of Immunology and Allergology in Otolaryngology (to R.K.).
Abbreviations used:
- IEC
intestinal epithelial cell
- IFNγ
interferon γ
- myr
myristoylation signal
- NLS
nuclear localization signal
- WT
wild type
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E13-09-0512) on July 30, 2014.
*These authors contributed equally to this work.
REFERENCES
- Brazil DP, Yang ZZ, Hemmings BA. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem Sci. 2004;29:233–242. doi: 10.1016/j.tibs.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Capaldo CT, Beeman N, Hilgarth RS, Nava P, Louis NA, Naschberger E, Sturzl M, Parkos CA, Nusrat A. IFN-gamma and TNF-alpha-induced GBP-1 inhibits epithelial cell proliferation through suppression of beta-catenin/TCF signaling. Mucosal Immunol. 2012;5:681–690. doi: 10.1038/mi.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson KC, Adams AM, Goodson JM, McDonald CE, Potter JC, Berndt JD, Biechele TL, Taylor RJ, Moon RT. Wnt/beta-catenin signaling promotes differentiation, not self-renewal, of human embryonic stem cells and is repressed by Oct4. Proc Natl Acad Sci USA. 2012;109:4485–4490. doi: 10.1073/pnas.1118777109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dihlmann S, Kloor M, Fallsehr C, von Knebel Doeberitz M. Regulation of AKT1 expression by beta-catenin/Tcf/Lef signaling in colorectal cancer cells. Carcinogenesis. 2005;26:1503–1512. doi: 10.1093/carcin/bgi120. [DOI] [PubMed] [Google Scholar]
- Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T, Lu Z. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007;282:11221–11229. doi: 10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto S, Hsieh CM, Maemura K, Layne MD, Yet SF, Lee KH, Matsui T, Rosenzweig A, Taylor WG, Rubin JS, et al. Akt participation in the Wnt signaling pathway through Dishevelled. J Biol Chem. 2001;276:17479–17483. doi: 10.1074/jbc.C000880200. [DOI] [PubMed] [Google Scholar]
- Gardino AK, Yaffe MB. 14–3-3 proteins as signaling integration points for cell cycle control and apoptosis. Semin Cell Dev Biol. 2011;22:688–695. doi: 10.1016/j.semcdb.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Sancho JM, Aguilera O, Garcia JM, Pendas-Franco N, Pena C, Cal S, Garcia de Herreros A, Bonilla F, Munoz A. The Wnt antagonist DICKKOPF-1 gene is a downstream target of beta-catenin/TCF and is downregulated in human colon cancer. Oncogene. 2005;24:1098–1103. doi: 10.1038/sj.onc.1208303. [DOI] [PubMed] [Google Scholar]
- He XC, Yin T, Grindley JC, Tian Q, Sato T, Tao WA, Dirisina R, Porter-Westpfahl KS, Hembree M, Johnson T, et al. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet. 2007;39:189–198. doi: 10.1038/ng1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvius M, Paulsson J, Weibrecht I, Leuchowius KJ, Andersson AC, Wahlby C, Gullberg M, Botling J, Sjoblom T, Markova B, et al. In situ detection of phosphorylated platelet-derived growth factor receptor beta using a generalized proximity ligation method. Mol Cell Proteomics. 2007;6:1500–1509. doi: 10.1074/mcp.M700166-MCP200. [DOI] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Sassano A, Joseph AM, Majchrzak-Kita B, Eklund EA, Verma A, Brachmann SM, Fish EN, Platanias LC. Dual regulatory roles of phosphatidylinositol 3-kinase in IFN signaling. J Immunol. 2008;181:7316–7323. doi: 10.4049/jimmunol.181.10.7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S, Nusrat A. The life and death of epithelia during inflammation: lessons learned from the gut. Annu Rev Pathol. 2012;7:35–60. doi: 10.1146/annurev-pathol-011811-120905. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- Laprise P, Chailler P, Houde M, Beaulieu JF, Boucher MJ, Rivard N. Phosphatidylinositol 3-kinase controls human intestinal epithelial cell differentiation by promoting adherens junction assembly and p38 MAPK activation. J Biol Chem. 2002;277:8226–8234. doi: 10.1074/jbc.M110235200. [DOI] [PubMed] [Google Scholar]
- Li FQ, Mofunanya A, Harris K, Takemaru K. Chibby cooperates with 14-3-3 to regulate beta-catenin subcellular distribution and signaling activity. J Biol Chem. 2008;181:1141–1154. doi: 10.1083/jcb.200709091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Kato Y, Zhang Z, Do VM, Yankner BA, He X. beta-Trcp couples beta-catenin phosphorylation-degradation and regulates Xenopus axis formation. Proc Natl Acad Sci USA. 1999;96:6273–6278. doi: 10.1073/pnas.96.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megidish T, Cooper J, Zhang L, Fu H, Hakomori S. A novel sphingosine-dependent protein kinase (SDK1) specifically phosphorylates certain isoforms of 14-3-3 protein. J Biol Chem. 1998;273:21834–21845. doi: 10.1074/jbc.273.34.21834. [DOI] [PubMed] [Google Scholar]
- Mitra A, Raychaudhuri SK, Raychaudhuri SP. IL-22 induced cell proliferation is regulated by PI3K/Akt/mTOR signaling cascade. Cytokine. 2012;60:38–42. doi: 10.1016/j.cyto.2012.06.316. [DOI] [PubMed] [Google Scholar]
- Naito AT, Akazawa H, Takano H, Minamino T, Nagai T, Aburatani H, Komuro I. Phosphatidylinositol 3-kinase-Akt pathway plays a critical role in early cardiomyogenesis by regulating canonical Wnt signaling. Circ Res. 2005;97:144–151. doi: 10.1161/01.RES.0000175241.92285.f8. [DOI] [PubMed] [Google Scholar]
- Nava P, Koch S, Laukoetter MG, Lee WY, Kolegraff K, Capaldo CT, Beeman N, Addis C, Gerner-Smidt K, Neumaier I, et al. Interferon-gamma regulates intestinal epithelial homeostasis through converging beta-catenin signaling pathways. Immunity. 2010;32:392–402. doi: 10.1016/j.immuni.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng SS, Mahmoudi T, Danenberg E, Bejaoui I, de Lau W, Korswagen HC, Schutte M, Clevers H. Phosphatidylinositol 3-kinase signaling does not activate the wnt cascade. J Biol Chem. 2009;284:35308–35313. doi: 10.1074/jbc.M109.078261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niida A, Hiroko T, Kasai M, Furukawa Y, Nakamura Y, Suzuki Y, Sugano S, Akiyama T. DKK1, a negative regulator of Wnt signaling, is a target of the beta-catenin/TCF pathway. Oncogene. 2004;23:8520–8526. doi: 10.1038/sj.onc.1207892. [DOI] [PubMed] [Google Scholar]
- Obsil T, Obsilova V. Structural basis of 14-3-3 protein functions. Semin Cell Dev Biol. 2011;22:663–672. doi: 10.1016/j.semcdb.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Powell DW, Rane MJ, Chen Q, Singh S, McLeish KR. Identification of 14-3-3zeta as a protein kinase B/Akt substrate. J Biol Chem. 2002;277:21639–21642. doi: 10.1074/jbc.M203167200. [DOI] [PubMed] [Google Scholar]
- Sato T, Maekawa S, Yasuda S, Domeki Y, Sueyoshi K, Fujiwara M, Fukao Y, Goto DB, Yamaguchi J. Identification of 14-3-3 proteins as a target of ATL31 ubiquitin ligase, a regulator of the C/N response in Arabidopsis. Plant J. 2011;68:137–146. doi: 10.1111/j.1365-313X.2011.04673.x. [DOI] [PubMed] [Google Scholar]
- Seno H, Miyoshi H, Brown SL, Geske MJ, Colonna M, Stappenbeck TS. Efficient colonic mucosal wound repair requires Trem2 signaling. Proc Natl Acad Sci USA. 2009;106:256–261. doi: 10.1073/pnas.0803343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluchanko NN, Gusev NB. Oligomeric structure of 14-3-3 protein: what do we know about monomers. FEBS Lett. 2012;586:4249–4256. doi: 10.1016/j.febslet.2012.10.048. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Daut J, Schwappach B. Membrane proteins as 14-3-3 clients in functional regulation and intracellular transport. Physiology (Bethesda) 2011;26:181–191. doi: 10.1152/physiol.00042.2010. [DOI] [PubMed] [Google Scholar]
- Soderberg O, Gullberg M, Jarvius M, Ridderstrale K, Leuchowius KJ, Jarvius J, Wester K, Hydbring P, Bahram F, Larsson LG, et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods. 2006;3:995–1000. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- Tian Q, Feetham MC, Tao WA, He XC, Li L, Aebersold R, Hood L. Proteomic analysis identifies that 14-3-3zeta interacts with beta-catenin and facilitates its activation by Akt. Proc Natl Acad Sci USA. 2004;101:15370–15375. doi: 10.1073/pnas.0406499101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q, He XC, Hood L, Li L. Bridging the BMP and Wnt pathways by PI3 kinase/Akt and 14-3-3zeta. Cell Cycle. 2005;4:215–216. [PubMed] [Google Scholar]
- Wang R, Brattain MG. AKT can be activated in the nucleus. Cell Signal. 2006;18:1722–1731. doi: 10.1016/j.cellsig.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Xie Z, Bikle DD. The recruitment of phosphatidylinositol 3-kinase to the E-cadherin-catenin complex at the plasma membrane is required for calcium-induced phospholipase C-gamma1 activation and human keratinocyte differentiation. J Biol Chem. 2007;282:8695–8703. doi: 10.1074/jbc.M609135200. [DOI] [PubMed] [Google Scholar]
- Xie Z, Singleton PA, Bourguignon LY, Bikle DD. Calcium-induced human keratinocyte differentiation requires src- and fyn-mediated phosphatidylinositol 3-kinase-dependent activation of phospholipase C-gamma1. Mol Biol Cell. 2005;16:3236–3246. doi: 10.1091/mbc.E05-02-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Shao Z, Kerkela R, Ichijo H, Muslin AJ, Pombo C, Force T. Serine 58 of 14-3-3zeta is a molecular switch regulating ASK1 and oxidant stress-induced cell death. Mol Cell Biol. 2009;29:4167–4176. doi: 10.1128/MCB.01067-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.